Abstract
Context
The majority of remaining tropical forests exist as fragments embedded in a matrix of agricultural production. Understanding the effects of these agricultural landscapes on species dispersal is crucial in the development of successful conservation planning.
Objective
The objective of this study was to examine the influence of five landscape features within a coffee agroecosystem (i.e., slope, elevation, streams, riparian effect, and tree cover) on Heteromys desmarestianus goldmani gene flow. We expected that landscape variables linked to more intense agricultural management (e.g., low tree cover, riparian effect) would reduce gene flow in H. d. goldmani.
Methods
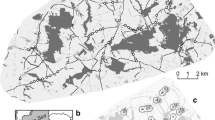
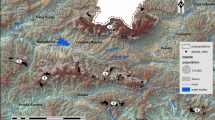
This study was conducted in a 4 km × 2 km area within the coffee growing region of Soconusco in Chiapas, Mexico. We used 12 microsatellite markers to calculate individual-based estimates of gene flow as a measure of dispersal. We used resistance surface modelling, using ResistanceGA (Peterman in Methods Ecol Evol 9:1638–1647, 2018) to identify if any of the landscape features analyzed explained the patterns of gene flow.
Results
Our results showed patterns of population structure and weak isolation-by-distance, as found previously for H. d. goldmani by Otero Jiménez et al. (Conserv Genet 19:495–499, 2018). Slope and tree cover were the two landscape features that could best explain gene flow patterns. More specifically, intermediate slopes and tree cover represent the lowest resistance to gene flow for H. d. goldmani and, thus, have a role in promoting gene flow in this species.
Conclusion
This study highlights the potential of integrating molecular and landscape data to explore population connectivity of elusive species, such as terrestrial small mammals. Our study adds to the growing body of literature in landscape genetics by demonstrating that a rodent species shows population structure at a small scale resulting from landscape factors linked to agricultural management.




Similar content being viewed by others
References
Blois JL, McGuire JL, Hadly EA (2010) Small mammal diversity loss in response to late-Pleistocene climatic change. Nature 465(7299):771–774
Bohdal T, Navrátil J, Sedláček F (2016) Small terrestrial mammals living along streams acting as natural landscape barriers. Ekologia (Bratislava) 35(2):191–204
Bolstad P (2016). GIS fundamentals. A first text on Geographic Information Systems (5th edition)
Brown JH, Heske EJ (1990) Control of a desert-grassland transition by a keystone rodent guild. Science 250(4988):1705–1707
Carver S (2010) Resistance of mammal assemblage structure to dryland salinity in a fragmented landscape. J R Soc West Aust 93:119–128
Caudill SA, Rice RA (2016) Do bird friendly® coffee criteria benefit mammals? Assessment of mammal diversity in Chiapas, Mexico. PLoS ONE 11(11):e0165662–e0165662
Clarke RT, Rothery P, Raybould AF (2002) Confidence limits for regression relationships between distance matrices: estimating gene flow with distance. J Agric Biol Environ Stat 7(3):361
Davidson AD, Lightfoot DC (2006) Keystone rodent interactions: prairie dogs and kangaroo rats structure the biotic composition of a desertified grassland. Ecography 29(5):755–765
DeMattia EA, Curran LM, Rathcke BJ (2004) Effects of small rodents and large mammals on Neotropical seeds. Ecology 85(8):2161–2170
Dickman CR (1999) Rodent-ecosystem relationships: a review. Ecologically-based management of rodent pests. ACIAR Monograph 59:113–133
ESRI (2015) ARCGIS. Environmental Systems Research Incorporated, Redlands
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
Fischer C, Thies C, Tscharntke T (2011) Small mammals in agricultural landscapes: opposing responses to farming practices and landscape complexity. Biol Cons 144(3):1130–1136
Fleming TH (1974) The population ecology of two species of Costa Rican Heteromyid rodents. Ecology 55(3):493–510
Fleming TH (1983) Heteromys desmarestianus. In: Janzen DH (ed) Costa Rican natural history. University of Chicago Press, Chicago, pp 474–475
Flores-Manzanero A, Luna-Bárcenas MA, Dyer RJ, Vázquez-Domínguez E (2018) Functional connectivity and home range inferred at a microgeographic landscape genetics scale in a desert-dwelling rodent. Ecol Evol 9(1):ece3.4762
Guillot G (2008) Inference of structure in subdivided populations at low levels of genetic differentiation–the correlated allele frequencies model revisited. Bioinformatics 24(19):2222–2228
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1(2):e1500052
Hooke RL, Martín-Duque JF, Pedraza J (2012) Land transformation by humans: a review. GSA Today 22:4–10
Iverson AL (2015) Biological control, biodiversity, and multifunctionality in coffee agroecosystems. (Unpublished PhD Dissertation) University of Michigan, Ann Arbor, Michigan, USA. https://hdl.handle.net/2027.42/113309
Jenson SK, Domingue JO (1988) Extracting topographic structure from digital elevation data for geographic information system analysis. Photogramm Eng Remote Sens 54(11):1593–1600
Keeley ATH, Beier P, Gagnon JW (2016) Estimating landscape resistance from habitat suitability: effects of data source and nonlinearities. Landsc Ecol 31(9):2151–2162
Klinger R (2007) Catastrophes, disturbances and density-dependence: population dynamics of the spiny pocket mouse (Heteromys desmarestianus) in a neotropical lowland forest. J Trop Ecol 23(05):507–518
Lomolino MV, Perault DR (2001) Island biogeography and landscape ecology of mammals inhabiting fragmented, temperate rain forests. Glob Ecol Biogeogr 10(2):113–132
Lynch M, Ritland K (1999a) Estimation of pairwise relatedness with molecular markers. Genetics 152(4):1753–1766
Martensen AC, Ribeiro MC, Banks-Leite C, Prado PI, Metzger JP (2012) Associations of forest cover, fragment area, and connectivity with neotropical understory bird species richness and abundance. Conserv Biol 26(6):1100–1111
Martínez-Gallardo R, Sánchez-Cordero V (1993) Dietary value of fruits and seeds to spiny pocket mice, Heteromys desmarestianus (Heteromyidae). J Mammal 74(2):436–442
Moguel P, Toledo VM (1999) Biodiversity conservation in traditional coffee systems of Mexico. Conserv Biol 13(1):11–21
Munshi-South J, Zolnik CP, Harris SE (2016) Population genomics of the Anthropocene: urbanization is negatively associated with genome-wide variation in white-footed mouse populations. Evol Appl 9(4):546–564
Otero Jiménez B, Vandermeer JH, Tucker PK (2018) Effect of coffee agriculture management on the population structure of a forest dwelling rodent (Heteromys desmarestianus goldmani). Conserv Genet 19(2):495–499
Oyler-McCance SJ, Fedy BC, Landguth EL (2013) Sample design effects in landscape genetics. Conserv Genet 14(2):275–285
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28(19):2537–2539
Perfecto I, Vandermeer J (2002) Quality of agroecological matrix in a tropical montane landscape: ants in coffee plantations in southern Mexico. Conserv Biol 16(1):174–182
Perfecto I, Vandermeer J (2008) Biodiversity conservation in tropical agroecosystems. Ann N Y Acad Sci 1134(1):173–200
Perfecto I, Vandermeer J, Wright A (2010) Nature's matrix: linking agriculture, conservation and food sovereignty. Routledge, London
Peterman W (2018) ResistanceGA: an R package for the optimization of resistance surfaces using genetic algorithms. Methods Ecol Evol 9:1638–1647
Peterman WE, Connette GM, Semlitsch RD, Eggert LS (2014) Ecological resistance surfaces predict fine-scale genetic differentiation in a terrestrial woodland salamander. Mol Ecol 23(10):2402–2413
Popescu VD, Hunter ML (2011) Clear-cutting affects habitat connectivity for a forest amphibian by decreasing permeability to juvenile movements. Ecol Appl 21(4):1283–1295
Rogers DS, González MW (2010) Phylogenetic relationships among spiny pocket mice (Heteromys) inferred from mitochondrial and nuclear sequence data. J Mammal 91(4):914–930
Rousset F (2000) Genetic differentiation between individuals. J Evol Biol 13(1):58–62
Rousset F (2008) Genepop'007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Res 8:103–106
Russo I-RM, Sole CL, Barbato M, von Bramann U, Bruford MW (2016) Landscape determinants of fine-scale genetic structure of a small rodent in a heterogeneous landscape (Hluhluwe-iMfolozi Park, South Africa). Sci Rep 6(1):29168–29168
Santos-Filho M, Peres CA, da Silva DJ, Sanaiotti TM (2012) Habitat patch and matrix effects on small-mammal persistence in Amazonian forest fragments. Biodivers Conserv 21(4):1127–1147
Savidge IR (1973) A stream as a barrier to homing in Peromyscus leucopus. J Mammal 54(4):982–984
van Etten J (2017) R package gdistance: distances and routes on geographical grid. J Stat Softw 76(13):1–21
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4(3):535–538
Van Strien MJ, Keller D, Holderegger R (2012) A new analytical approach to landscape genetic modelling: least-cost transect analysis and linear mixed models. Mol Ecol 21(16):4010–4023
Wolff JO (1999) Behavioral model systems. In: Barrett GW, Peles JD (eds) Landscape ecology of small mammals. Springer-Verlag, New York, pp 11–40
Wright S (1951) The genetical structure of populations. Ann Eugen 15(4):323–354
Zeller KA, McGarigal K, Whiteley AR (2012) Estimating landscape resistance to movement: review. Landsc Ecol 27(6):777–797
Acknowledgements
We thank Dr. Consuelo Lorenzo and El Colegio de la Frontera Sur-San Cristobal in Chiapas, Mexico for their help in sample collection. Thanks to Dr. Carlos J. Anderson for his valuable comments on the manuscript and support with statistical analysis and Dr. M. Raquel Marchan Rivadeneira for assistance with genetic analysis. We thank the managers, farmers and owners of Finca Irlanda and Finca Hamburgo in Chiapas, Mexico for allowing us to conduct this study and for their support with fieldwork. This work received financial support from the University of Michigan Center for Latin America and Caribbean Studies Tinker Field Research Grant and the University of Michigan Rackham Graduate School. Beatriz Otero Jiménez was supported in part by University of Michigan Genetics Training Program (T32-GM07544).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Otero Jiménez, B., Li, K. & Tucker, P.K. Landscape drivers of connectivity for a forest rodent in a coffee agroecosystem. Landscape Ecol 35, 1249–1261 (2020). https://doi.org/10.1007/s10980-020-00999-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-020-00999-6