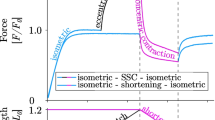
Abstract
Double mutation D208Q:K450L was introduced in the beta isoform of human cardiac myosin to remove the salt bridge D208:K450 connecting loop 1 and the seven-stranded beta sheet within the myosin head. Beta isoform-specific salt bridge D208:K450, restricting the flexibility of loop 1, was previously discovered in molecular dynamics simulations. Earlier it was proposed that loop 1 modulates nucleotide affinity to actomyosin and we hypothesized that the electrostatic interactions between loop 1 and myosin head backbone regulate ATP binding to and ADP dissociation from actomyosin, and therefore, the time of the strong actomyosin binding. To examine the hypothesis we expressed the wild type and mutant of the myosin head construct (1–843 amino acid residues) in differentiated C2C12 cells, and the kinetics of ATP-induced actomyosin dissociation and ADP release were characterized using stopped-flow spectrofluorometry. Both constructs exhibit a fast rate of ATP binding to actomyosin and a slow rate of ADP dissociation, showing that ADP release limits the time of the strongly bound state of actomyosin. We observed a faster rate of ATP-induced actomyosin dissociation with the mutant, compared to the wild type actomyosin. The rate of ADP release from actomyosin remains the same for the mutant and the wild type actomyosin. We conclude that the flexibility of loop 1 is a factor affecting the rate of ATP binding to actomyosin and actomyosin dissociation. The flexibility of loop 1 does not affect the rate of ADP release from human cardiac actomyosin.





Similar content being viewed by others
Data availability
Raw data generated during the current study are available from the corresponding author on reasonable request. All analyzed data are included in this published article and its supplementary information file.
Code availability
Origin (OriginLab Corp, Northampton, MA) and Wolfram Mathematica (Champaign, IL) were used in data analysis. Wolfram Mathematica scripts are available in the supplementary information file.
References
Clark R, Ansari MA, Dash S, Geeves MA, Coluccio LM (2005) Loop 1 of transducer region in mammalian class I myosin, Myo1b, modulates actin affinity, ATPase activity, and nucleotide access. J Biol Chem 280(35):30935–30942
Cowan JA (1991) Metallobiochemistry of magnesium. Coordination complexes with biological substrates: site specificity, kinetics and thermodynamics of binding, and implications for activity. Inorg Chem 2740–2747:2740–2747
Deacon JC, Bloemink MJ, Rezavandi H, Geeves MA, Leinwand LA (2012) Identification of functional differences between recombinant human alpha and beta cardiac myosin motors. Cell Mol Life Sci 69(13):2261–2277
Fisher AJ, Smith CA, Thoden JB, Smith R, Sutoh K, Holden HM, Rayment I (1995) X-ray structures of the myosin motor domain of Dictyostelium discoideum complexed with MgADP.BeFx and MgADP.AlF4. Biochemistry 34(28):8960–8972
Furch M, Geeves MA, Manstein DJ (1998) Modulation of actin affinity and actomyosin adenosine triphosphatase by charge changes in the myosin motor domain. Biochemistry 37(18):6317–6326
Gargey A, Ge J, Tkachev YV, Nesmelov YE (2019) Electrostatic interactions in the force-generating region of the human cardiac myosin modulate ADP dissociation from actomyosin. Biochem Biophys Res Commun 509(4):978–982
Gargey A, Iragavarapu SB, Grdzelishvili AV, Nesmelov YE (2021) Electrostatic interactions in the SH1-SH2 helix of human cardiac myosin modulate the time of strong actomyosin binding. J Muscle Res Cell Motil 42(2):137–147
Gulick AM, Bauer CB, Thoden JB, Rayment I (1997) X-ray structures of the MgADP, MgATPgammaS, and MgAMPPNP complexes of the Dictyostelium discoideum myosin motor domain. Biochemistry 36(39):11619–11628
Houk TW Jr., Ue K (1974) The measurement of actin concentration in solution: a comparison of methods. Anal Biochem 62(1):66–74
Lexell J, Jarvis JC, Currie J, Downham DY, Salmons S (1994) Fibre type composition of rabbit tibialis anterior and extensor digitorum longus muscles. J Anat 185(Pt 1):95–101
Margossian SS, Lowey S (1982) Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 85:55–71
Murphy CT, Spudich JA (1998) Dictyostelium myosin 25–50K loop substitutions specifically affect ADP release rates. Biochemistry 37(19):6738–6744
Murphy CT, Spudich JA (2000) Variable surface loops and myosin activity: accessories to a motor. J Muscle Res Cell Motil 21(2):139–151
Pereira JS, Pavlov D, Nili M, Greaser M, Homsher E, Moss RL (2001) Kinetic differences in cardiac myosins with identical loop 1 sequences. J Biol Chem 276(6):4409–4415
Reubold TF, Eschenburg S, Becker A, Kull FJ, Manstein DJ (2003) A structural model for actin-induced nucleotide release in myosin. Nat Struct Biol 10(10):826–830
Spudich JA (1994) How molecular motors work. Nature 372(6506):515–518
Strzelecka-Golaszewska H, Prochniewicz E, Nowak E, Zmorzynski S, Drabikowski W (1980) Chicken-gizzard actin: polymerization and stability. Eur J Biochem 104(1):41–52
Sweeney HL, Rosenfeld SS, Brown F, Faust L, Smith J, Xing J, Stein LA, Sellers JR (1998) Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem 273(11):6262–6270
Takagi Y, Yang Y, Fujiwara I, Jacobs D, Cheney RE, Sellers JR, Kovacs M (2008) Human myosin Vc is a low duty ratio, nonprocessive molecular motor. J Biol Chem 283(13):8527–8537
Waller GS, Ouyang G, Swafford J, Vibert P, Lowey S (1995) A minimal motor domain from chicken skeletal muscle myosin. J Biol Chem 270(25):15348–15352
Acknowledgements
We thank the anonymous reviewers for critical reviews of an earlier version of the manuscript.
Funding
This work was supported by the National Institutes of Health, Grant No. HL132315.
Author information
Authors and Affiliations
Contributions
YN designed research, AG and YN performed research and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
Actin was produced from rabbit skeletal tissue. All experimental protocols were approved by the Institutional Animal Care and Use Committee of UNC Charlotte and all experiments were performed in accordance with relevant guidelines and regulations.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gargey, A., Nesmelov, Y.E. Electrostatic interaction of loop 1 and backbone of human cardiac myosin regulates the rate of ATP induced actomyosin dissociation. J Muscle Res Cell Motil 43, 1–8 (2022). https://doi.org/10.1007/s10974-021-09611-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-021-09611-z