Abstract
The technique of selective removal of the thin filament by gelsolin in bovine cardiac muscle fibres, and reconstitution of the thin filament from isolated proteins is reviewed, and papers that used reconstituted preparations are discussed. By comparing the results obtained in the absence/presence of regulatory proteins tropomyosin (Tm) and troponin (Tn), it is concluded that the role of Tm and Tn in force generation is not only to expose the binding site of actin to myosin, but also to modify actin for better stereospecific and hydrophobic interaction with myosin. This conclusion is further supported by experiments that used a truncated Tm mutant and the temperature study of reconstituted fibres. The conclusion is consistent with the hypothesis that there are three states in the thin filament: blocked state, closed state, and open state. Tm is the major player to produce these effects, with Tn playing the role of Ca2+ sensing and signal transmission mechanism. Experiments that changed the number of negative charges at the N-terminal finger of actin demonstrates that this part of actin is essential to promote the strong interaction between actin and myosin molecules, in addition to the well-known weak interaction that positions the myosin head at the active site of actin prior to force generation.
Similar content being viewed by others
References
Abbott RH, Steiger GJ (1977) Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol (Lond) 266:13–42
Andrews MA, Maughan DW, Nosek TM, Godt RE (1991) Ion-specific and general ionic effects on contraction of skinned fast-twitch skeletal muscle from the rabbit. J Gen Physiol 98:1105–1125
Araujo A, Walker JW (1996) Phosphate release and force generation in cardiac myocytes investigated with caged phosphate and caged calcium. Biophys J 70:2316–2326
Bagshaw CR, Trentham DR (1974) The characterization of myosin-product complexes and of product-release steps during the magnesium ino-dependent adenosine triphosphatase reaction. Biochem J 141:331–349
Bershitsky SY, Tsaturyan AK (1992) Tension responses to joule temperature jump in skinned rabbit muscle fibres. J Physiol (Lond) 447:425–448
Bing W, Knott A, Marston SB (2000) A simple method for measuring the relative force exerted by myosin on actin filaments in the in vitro motility assay: evidence that tropomyosin and troponin increase force in single thin filaments. Biochem J 350:693–699
Blanchard E, Seidman C, Seidman JG, LeWinter M, Maughan D (1999) Altered crossbridge kinetics in the αMHC403/+ mouse model of familial hypertrophic cardiomyopathy. Circ Res 84:475–483
Blanchard EM, Smith GL, Allen DG, Alpert NR (1990) The effects of 2,3-butanedione monoxime on initial heat, tension, and aequorin light output of ferret papillary muscles. Pflugers Arch 416:219–221
Brenner B, Eisenberg E (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83:3542–3546
Brenner B, Schoenberg M, Chalovich JM, Greene LE, Eisenberg E (1982) Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci USA 79:7288–7291
Chalovich JM (1992) Actin mediated regulation of muscle contraction. Pharmacol Ther 55:95–148
Chang AN, Potter JD (2005) Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev 10:225–235
Cook RK, Root D, Miller C, Reisler E, Rubenstein PA (1993) Enhanced stimulation of myosin subfragment 1 ATPase activity by addition of negatively charged residues to the yeast actin NH2 terminus. J Biol Chem 268:2410–2415
Coupland ME, Puchert E, Ranatunga KW (2001) Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol (Lond) 536:879–891
Dantzig J, Goldman Y, Millar NC, Lacktis J, Homsher E (1992) Reversal of the cross-bridge force-generation transition by the photogeneration of phosphate in rabbit psoas muscle fibers. J Physiol (Lond) 451:247–278
DasGupta G, Reisler E (1989) Antibody against the amino terminus of alpha-actin inhibits actomyosin interactions in the presence of ATP. J Mol Biol 207:833–836
Edman KAP (1979) The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol (Lond) 269:255–272
Fortune NS, Geeves MA, Ranatunga KW (1991) Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc Natl Acad Sci USA 88:7323–7327
Fowler VM, Sussmann MA, Miller PG, Flucher BE, Daniels MP (1993) Tropomodulin is associated with the free (pointed) ends of the thin filaments in rat skeletal muscle. J Cell Biol 120:411–420
Fujita H, Ishiwata S (1998) Spontaneous oscillatory contraction without regulatory proteins in actin filament-reconstituted fibers. Biophys J 75:1439–1445
Fujita H, Ishiwata S (1999) Tropomyosin modulates pH dependence of isometric tension. Biophys J 77:1540–1546
Fujita H, Kawai M (2002) Temperature effect on isometric tension is mediated by regulatory proteins tropomyosin and troponin in bovine myocardium. J Physiol (Lond) 539:267–276
Fujita H, Lu X, Suzuki M, Ishiwata S, Kawai M (2004) The effect of tropomyosin on force and elementary steps of the cross-bridge cycle in reconstituted bovine myocardium. J Physiol (Lond) 556:637–649
Fujita H, Sasaki D, Ishiwata S, Kawai M (2002) Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J 82:915–928
Fujita H, Yasuda K, Niitsu S, Funatsu T, Ishiwata S (1996) Structural and functional reconstitution of thin filaments in the contractile apparatus of cardiac muscle. Biophys J 71:2307–2318
Funatsu T, Anazawa T, Ishiwata S (1994) Structural and functional reconstitution of thin filaments in skeletal muscle. J Muscle Res Cell Motil 15:158–171
Funatsu T, Asami Y, Ishiwata S (1988) β-Actinin: a capping protein at the pointed end of thin filaments in skeletal muscle. J Biochem (Tokyo) 103:61–71
Funatsu T, Higuchi H, Ishiwata S (1990) Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol 110:53–62
Funatsu T, Kono E, Higuchi H, Kimura S, Ishiwata S, Yoshioka T, Maruyama K, Tsukita S (1993) Elastic filaments in situ in cardiac muscle: deep-etch replica analysis in combination with selective removal of actin and myosin filaments. J Cell Biol 120:711–724
Furch M, Geeves MA, Manstein DJ (1998) Modulation of actin affinity and actomyosin adenosine triphosphatase by charge changes in the myosin motor domain. Biochemistry 37:6317–6326
Galler S, Wang BG, Kawai M (2005) Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J 89:3248–3260
Geeves MA, Goody RS, Gutfreund H (1984) Kinetics of acto-S1 interaction as a guide to a model for the cross-bridge cycle. J Muscle Res Cell Motil 5:351–361
Goldman YE, Hibberd MG, Trentham DR (1984) Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5′-triphosphate. J Physiol (Lond) 354:577–604
Goldman YE, McCray JA, Ranatunga KW (1987) Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J Physiol (Lond) 392:71–95
Gordon AM, Chen Y, Liang B, LaMadrid M, Luo Z, Chase PB (1998) Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol 453:187–196
Heinl P, Kuhn HJ, Rüegg JC (1974) Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol (Lond) 237:243–258
Herrmann C, Wray J, Travers F, Barman T (1992) Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry 31:12227–12232
Highsmith S (1977) The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys 180:404–408
Higuchi H, Yanagida T, Goldman YE (1995) Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 69:1000–1010
Hitchcock-DeGregori SE, An Y (1996) Integral repeats and a continuous coiled coil are required for binding of striated muscle tropomyosin to the regulated actin filament. J Biol Chem 16:3600–3603
Hitchcock-DeGregori SE, Varnell TA (1990) Tm has discrete actin-binding sites with sevenfold and fourteenfold periodicities. J Mol Biol 214:885–896
Holmes KC, Schroder RR, Sweeney HL, Houdusse A (2004) The structure of the rigor complex and its implications for the power stroke. Phil Trans Roy Soc Lond B Biol Sci 359:1819–1828
Homsher E, Lee DM, Morris C, Pavlov D, Tobacman LS (2000) Regulation of force and unloaded sliding speed in single thin filaments: effects of regulatory proteins and calcium. J Physiol (Lond) 524:233–243
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Chem 7:255–318
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Huxley HE, Stewart A, Sosa H, Irving T (1994) X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J 67:2411–2421
Ishiwata S (1973) A study on the F-actin, tropomyosin and troponin complex. I. Gel-filament transformation. Biochim Biophys Acta 303:77–89
Ishiwata S, Funatsu T (1985) Does actin bind to the ends of thin filaments in skeletal muscle?. J Cell Biol 100:282–291
Ishiwata S, Funatsu T, Fujita H (1998) Contractile properties of thin (actin) filament-reconstituted muscle fibers. Adv Exp Med Biol 453:319–329
Ishiwata S, Kondo H (1978) Studies on the F-actin–tropomyosin–troponin complex. II. Partial reconstitution of thin filament by F-actin, tropomyosin and tropomyosin binding component of troponin (TNT). Biochim Biophys Acta 534:341–349
Ishiwata S, Manuck BA, Seidel JC, Gergely J (1986) Saturation transfer electron paramagnetic resonance study of the mobility of myosin heads in myofibrils under conditions of partial dissociation. Biophys J 49:821–828
Joel PB, Trybus KM, Sweeney HL (2001) Two conserved lysines at the 50/20-kda junction of myosin are necessary for triggering actin activation. J Biol Chem 276:2998–3003
Kawai M (2003) What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres?. J Muscle Res Cell Motil 24:127–138
Kawai M, Brandt PW (1980) Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1:279–303
Kawai M, Halvorson HR (1991) Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J 59:329–342
Kawai M, Kido T, Vogel M, Fink RH, Ishiwata S (2006) Temperature change does not affect force between regulated actin filaments and HMM in single molecule experiments. J Physiol (Lond) 574(pt 3):877–887
Kawai M, Saeki Y, Zhao Y (1993) Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res 73:35–50
Kawai M, Zhao Y (1993) Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibers. Biophys J 65:638–651
Kitamura K, Tokunaga M, Iwane AH, Yanagida T (1999) A single myosin head moves along an actin filament with regular steps of 5.3 nanometres. Nature 397:129–134
Kurokawa H, Fujii W, Ohmi K, Sakurai T, Nonomura Y (1990) Simple and rapid purification of brevin. Biochem Biophys Res Commun 168:451–457
Landis CA, Bobkova A, Homsher E, Tobacman LS (1997) The active state of the thin filament is destabilized by an internal deletion in tropomyosin. J Biol Chem 272:14051–14056
Littlefield R, Fowler VM (1998) Defining actin filament length in striated muscle: rulers and caps or dynamic stability?. Annu Rev Cell Dev Biol 14:487–525
Lu X, Bryant MK, Bryan KE, Rubenstein PA, Kawai M (2005) Role of the N-terminal negative charges of actin in force generation and cross-bridge kinetics in reconstituted bovine cardiac muscle fibres. J Physiol (Lond) 564:65–82
Lu X, Heeley DH, Smillie LB, Kawai M (2006) Effects of tropomyosin (Tm) isoforms and phosphorylation on isometric tension and cross-bridge kinetics in bovine myocardium. Biophys J 90:120a (Abstr #558)
Lu X, Tobacman LS, Kawai M (2003) Effects of tropomyosin internal deletion Delta23Tm on isometric tension and the cross-bridge kinetics in bovine myocardium. J Physiol (Lond) 553.2:457–471
Maruyama K, Natori R, Nonomura Y (1976) New elastic protein from muscle. Nature 262:58–60
McKillop DFA, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701
Metzger JM, Greaser ML, Moss RL (1989) Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol 93:855–883
Moncman CL, Wang K (1995) Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton 32:205–225
Murphy KP, Zhao Y, Kawai M (1996) Molecular forces involved in force generation during skeletal muscle contraction. J Exp Biol 199:2565–2571
Oosawa F, Fujime S, Ishiwata S, Mihashi K (1972) Dynamic property of F-actin and thin filament. Cold Spr Harb Symp Quant Biol 37:277–285
Poggesi C, Tesi C, Stehle R (2005) Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch 449:505–517
Pringle JWS (1967) The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol 17:1–60
Ranatunga KW (1999) Endothermic force generation in skinned cardiac muscle from rat. J Muscle Res Cell Motil 20:489–496
Ranatunga KW, Sharpe B, Turnbull B (1987) Contraction of human skeletal muscle at different temperatures. J Physiol (Lond) 390:383–395
Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA (1993) Structure of the actin–myosin complex and its implications for muscle contraction. Science 261:58–65
Reuben JP, Brandt PW, Berman M, Grundfest H (1971) Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol 57:385–407
Stehle R, Telley IA, Pfitzer G (2005) Transient kinetics in force and indivisual sarcomere lengths induced by phosphate. Biophys J 88:127a (Abstr #624)
Sutoh K (1982a) Identification of myosin-binding sites on the actin sequence. Biochemistry 21:3654–3661
Sutoh K (1982b) An actin-binding site on the 20K fragment of myosin subfragment 1. Biochemistry 21:4800–4804
Sutoh K, Ando M, Sutoh K, Toyoshima YY (1991) Site-directed mutations of Dictyostelium actin: disruption of a negative charge cluster at the N terminus. Proc Natl Acad Sci USA 88:7711–7714
Taylor EW (1979) Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem 6:103–164
Tobacman LS, Butters CA (2000) A new model of cooperative myosin-thin filament binding. J Biol Chem 275:27587–27593
Tonomura Y, Tokura S, Sekiya K (1962) Binding of myosin A to F-actin. J Biol Chem 237:1074–1081
Van Buren P, Palmiter KA, Warshaw DM (1999) Tropomyosin directly modulates ctomyosin mechanical performance at the level of a single actin filament. Proc Natl Acad Sci USA 96:12488–12493
Vandekerckhove J, Weber K (1978) At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol 126:783–802
Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J 67:2422–2435
Wang G, Kawai M (1997) Force generation and phosphate release steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys J 73:878–894
Wang G, Kawai M (2001) Effect of temperature on elementary steps of the cross-bridge cycle in rabbit soleus slow-twitch muscle fibres. J Physiol (Lond) 531:219–234
Wang K, McClure J, Tu A (1979) Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci USA 76:3698–3702
Wang K, Wright J (1988) Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol 107:2199–2212
Wannenburg T, Heijne GH, Geerdink JH, Van den Dool HW, Janssen PML, de Tombe PP (2000) Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. Am J Physiol 279:H779–H790
White DCS, Thorson J (1974) The kinetics of muscle contraction. Prog Biophys Mol Biol 27:173–255
Wolska BM, Wieczorek DF (2003) The role of tropomyosin in the regulation of myocardial contraction and relaxation. Pflugers Arch 446:1–8
Zhao Y, Kawai M (1994a) Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biosphys J 67:1655–1668
Zhao Y, Kawai M (1994b) BDM affects nucleotide binding and force generation steps of the cross-bridge cycle in rabbit psoas muscle fibres. Am J Physiol (Cell Physiol 35) 266:C437–C447
Zhao Y, Kawai M (1996) Inotropic agent EMD-53998 weakens nucleotide and phosphate binding to cross bridges in porcine myocardium. Am J Physiol (Heart Circ Physiol 40) 271:H1394–H1406
Zhao Y, Swamy PMG, Humphries KA, Kawai M (1996) The effect of partial extraction of troponin C on the elementary steps of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J 71:2759–2773
Acknowledgments
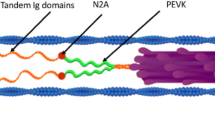
We would like to thank Dr. Hideaki Fujita for drawing Fig. 1, and to Dr. Madoka Suzuki and Ms. Kristen Stanton for critical reading of the manuscript. This work was supported in part by an NIH grant HL70041 to MK, and by Grants-in-Aid for Specially Promoted Research and for the 21st Century COE program (Physics of Self-organization Systems), and by “Establishment of Consolidated Research Institute for Advanced Science and Medical Care” from the Ministry of Education, Sports, Culture, Science and Technology of Japan to SI. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of awarding organizations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawai, M., Ishiwata, S. Use of thin filament reconstituted muscle fibres to probe the mechanism of force generation. J Muscle Res Cell Motil 27, 455–468 (2006). https://doi.org/10.1007/s10974-006-9075-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-006-9075-4