Abstract
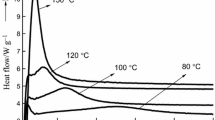
Highly branched polyurea (HBPU) resins have been developed as wood adhesives even though their cure kinetics was not well understood. Here, we report thermal cure kinetics of HBPU resins at three molar ratios (2.0, 2.5, and 3.0), using differential scanning calorimetry. A main endothermic peak at 200–240 °C of HBPU resins was used to calculate their thermal cure kinetics with several model-free kinetics methods such as Friedman (FR), Flynn–Wall–Ozawa (FWO), and Kissinger–Akahira–Sunose (KAS) method. Furthermore, Málek method was also used to predict an optimal reaction model. Thermal curing of HBPU resins changed from chemical-controlled reaction at low molar ratio to diffusion controlled one at high molar ratio, as the molar ratio of HBPU resins increased. FWO method was suitable for calculating the activation energy of 2.0 HBPU resins, while FR method was suitable for 2.5 and 3.0 HBPU resins. Furthermore, Málek method revealed that all of HBPU resins apparently followed autocatalytic reaction model, regardless of molar ratios. In addition, FWO and KAS method was appropriate to predict the reaction model of 2.0 and 3.0 HBPU resins with Málek method.











Similar content being viewed by others
References
Pizzi A, Mittal KL. Handbook of Adhesive Technology. 2nd ed., A. Pizzi and K.L. Mittal, 2003, Marcel Dekker, Inc., New York
Jiang S, Wang S, Du G, Hu M, Qian Z, Shi Z, et al. Highly branched polyurea-based resins: clean synthesis and wood bonding performance. ACS Sustain Chem Eng Am Chem Soc (ACS). 2022;10:7205–14. https://doi.org/10.1021/acssuschemeng.2c01916.
Park S, Park B-D. Crystallinity of low molar ratio urea-formaldehyde resins modified with cellulose nanomaterials. J Korean Wood Sci Technol. 2021;49:0–2. https://doi.org/10.5658/WOOD.2021.49.2.169.
Park S, Park B-D. Sustainable bio-based dialdehyde cellulose for transforming crystalline urea–formaldehyde resins into amorphous ones to improve their performance. J Ind Eng Chem. 2022. https://doi.org/10.1016/j.jiec.2022.05.012.
Dunky M. Urea–formaldehyde (UF) adhesive resins for wood. Int J Adhes Adhes. 1998;18:95–107. https://doi.org/10.1016/S0143-7496(97)00054-7.
Myers GE. Mechanisms of formaldehyde release from bonded wood products. ACS Symp Ser. 1986. https://doi.org/10.1021/bk-1986-0316.ch008.
Park BD, Jeong HW. Hydrolytic stability and crystallinity of cured ureaformaldehyde resin adhesives with different formaldehyde/urea mole ratios. Int J Adhes Adhes. 2011;31:524–9. https://doi.org/10.1016/j.ijadhadh.2011.05.001.
Park S, Jeong B, Park BD. A comparison of adhesion behavior of urea-formaldehyde resins with melamine-urea-formaldehyde resins in bonding wood. Forest. 2021;12(8):1037. https://doi.org/10.3390/f12081037.
Wibowo ES, Park B, Causin V. Recent advances in urea–formaldehyde resins: converting crystalline thermosetting polymers back to amorphous ones. Polym Rev. 2021. https://doi.org/10.1080/15583724.2021.2014520.
Wibowo ES, Park BD, Causin V. Hydrogen-bond-induced crystallization in low-molar-ratio urea-formaldehyde resins during synthesis. Ind Eng Chem Res. 2020;59:13095–104. https://doi.org/10.1021/acs.iecr.0c02268.
Wang H, Cao M, Li TH, Yang L, Duan ZG, Zhou XJ, Du GB. Characterization of the low molar ratio urea-formaldehyde resin with C-13 NMR and ESI-MS: negative effects of the post-added urea on the urea-formaldehyde polymers. Polymers (Basel). 2018;10:602. https://doi.org/10.3390/polym10060602.
Wibiwo ES, Park BD. Enhancing adhesion of thermosetting urea-formaldehyde resins by preventing the formation of H-bonds with multi-reactive melamine. J Adhes. 2020. https://doi.org/10.1080/00218464.2020.1830069.
Wibowo ES, Lubis MAR, Park B-D, Kim JS, Causin V. Converting crystalline thermosetting urea–formaldehyde resins to amorphous polymer using modified nanoclay. J Ind Eng Chem. 2020;87:78–89. https://doi.org/10.1016/j.jiec.2020.03.014.
Paiva NT, Henriques A, Cruz P, Ferra JM, Carvalho LH, Magalhães FD. Production of melamine fortified urea-formaldehyde resins with low formaldehyde emission. J Appl Polym Sci. 2012;124:2311–7. https://doi.org/10.1002/app.35282.
Yang H, Du G, Li Z, Ran X, Zhou X, Li T, et al. Superstrong adhesive of isocyanate-free polyurea with a branched structure. ACS Appl Polym Mater. 2021;3:1638–51. https://doi.org/10.1021/acsapm.1c00056.
Li T, Zhang B, Jiang S, Zhou X, Du G, Wu Z, et al. Novel highly branched polymer wood adhesive resin. ACS Sustain Chem Eng. 2020;8:5209–16. https://doi.org/10.1021/acssuschemeng.9b07732.
Voit BI, Lederer A. Hyperbranched and highly branched polymer architectures-synthetic strategies and major characterization aspects. Chem Rev. 2009;109:5924–73. https://doi.org/10.1021/cr900068q.
Mu B, Liu T, Tian W. Long-chain hyperbranched polymers: synthesis, properties, and applications. Macromol Rapid Commun. 2019;40:1800471. https://doi.org/10.1002/marc.201800471.
Xu Z, Liang Y, Ma X, Chen S, Yu C, Wang Y, et al. Recyclable thermoset hyperbranched polymers containing reversible hexahydro-s-triazine. Nat Sustain. 2020;3:29–34. https://doi.org/10.1038/s41893-019-0444-6.
Jiang S, Hu M, Du G, Duan Z, Zhou X, Li T. Highly branched polyurea-enhanced urea-formaldehyde resin. ACS Appl Polym Mater. 2021;3:1157–70. https://doi.org/10.1021/acsapm.0c01362.
Lv JY, Meng Y, He LF, Li XY, Wang HQ. Synthesis of a hyperbranched polyether epoxy through onestep proton transfer polymerization and its application as a toughener for epoxy resin dgeba. Chinese J Polym Sci. 2012;30:493–502. https://doi.org/10.1007/s10118-012-1166-7.
Zhao L, Hu X. Autocatalytic curing kinetics of thermosetting polymers: a new model based on temperature dependent reaction orders. Polymer (Guildf). 2010;51:3814–20. https://doi.org/10.1016/j.polymer.2010.05.056.
Zheng T, Wang X, Lu C, Zhang X, Ji Y, Bai C, et al. Studies on curing kinetics and tensile properties of silica-filled phenolic amine/epoxy resin nanocomposite. Polymers (Basel). 2019;11:680. https://doi.org/10.3390/polym11040680.
Thomas R, Durix S, Sinturel C, Omonov T, Goossens S, Groeninckx G, et al. Cure kinetics, morphology and miscibility of modified DGEBA-based epoxy resin—effects of a liquid rubber inclusion. Polymer (Guildf). 2007;48:1695–710. https://doi.org/10.1016/j.polymer.2007.01.018.
Kandelbauer A, Wuzella G, Mahendran A, Taudes I, Widsten P. Model-free kinetic analysis of melamine-formaldehyde resin cure. Chem Eng J. 2009;152:556–65. https://doi.org/10.1016/j.cej.2009.05.027.
Wibowo ES, Park BD. Cure kinetics of low-molar-ratio urea-formaldehyde resins reinforced with modified nanoclay using different kinetic analysis methods. Thermochim Acta. 2020;686:178552. https://doi.org/10.1016/j.tca.2020.178552.
Hatakeyama T, Quinn FX. Thermal analysis: fundamentals and applications to polymer science. Chichester: Wiley; 1994.
Thomas LC. Use of multiple heating rate DSC and modulated temperature DSC to detect and analyze temperature-time-dependent transitions in materials. Am Lab. 2001;33(1):262830–1.
Pal AK, Katiyar V. Theoretical and analyzed data related to thermal degradation kinetics of poly (L-lactic acid)/chitosan-grafted-oligo L-lactic acid (PLA/CH-g-OLLA) bionanocomposite films. Data Br. 2017;10:304–11. https://doi.org/10.1016/j.dib.2016.11.100.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68. https://doi.org/10.1016/S0040-6031(99)00253-1.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6. https://doi.org/10.1246/bcsj.38.1881.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C Polym Symp. 2007;6:183–95. https://doi.org/10.1002/polc.5070060121.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand Sect A: Phys Chem. 1966;70A:487.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6. https://doi.org/10.1021/ac60131a045.
Akahira T, Sunose T. Joint convention of four electrical institutes. Sci Educ Technol. 1971;16:22–31. https://doi.org/10.1021/i200014a015.
Vyazovkin S. Modification of the Integral Isoconversional Method to Account for Variation in the Activation Energy. J Comput Chem. 2001;22:178–83. https://doi.org/10.1002/1096-987X(20010130)22:2%3c178::AID-JCC5%3e3.0.CO;2-%23.
Golikeri SV, Luss D. Analysis of activation energy of grouped parallel reactions. AIChE J. 1972;18:277–82. https://doi.org/10.1002/aic.690180205.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6:639–42. https://doi.org/10.1002/app.1962.070062406.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7. https://doi.org/10.1007/BF01903696.
Redemann CE, Riesenfeld FC, La Viola FS. Formation of biuret from urea. Ind Eng Chem. 1958;50:633–6. https://doi.org/10.1021/ie50580a035.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32. https://doi.org/10.1002/marc.200600404.
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejón A, Criado JM. Limitations of model-fitting methods for kinetic analysis: polystyrene thermal degradation. Resour Conserv Recycl. 2013;74:75–81. https://doi.org/10.1016/j.resconrec.2013.02.014.
Vyazovkin S, Sbirrazzuoli N. Effect of viscosity on the kinetics of initial cure stages. Macromol Chem Phys. 2000;201(2):199–203. https://doi.org/10.1002/(SICI)1521-3935(20000201)201:2%3c199::AID-MACP199%3e3.0.CO;2-N.
Vyazovkin S, Mititelu A, Sbirrazzuoli N. Kinetics of epoxy-amine curing accompanied by the formation of liquid crystalline structure. Macromol Rapid Commun. 2003;24:1060–5. https://doi.org/10.1002/marc.200300023.
Khanna C, Chanda M. Kinetics of anhydride curing of lsophthalic diglycidyl ester using differential scanning calorimetry. J Appl Polym Sci. 1993;49(2):319–29. https://doi.org/10.1002/app.1993.070490212.
Gao W, Cao J-Z, Li J-Z. Effect of ammonium pentaborate on curing of aqueous phenol formaldehyde resin. Polym J. 2010;19(4):255–64.
Lubis MAR, Park BD. Influence of initial molar ratios on the performance of low molar ratio urea-formaldehyde resin adhesives. J Korean Wood Sci Technol. 2020;48:136–53. https://doi.org/10.5658/WOOD.2020.48.2.136.
Shojaei B, Najafi M, Yazdanbakhsh A, Abtahi M, Zhang C. A review on the applications of polyurea in the construction industry. Polym Adv Technol. 2021;32:2797–812. https://doi.org/10.1002/pat.5277.
Málek J, Mitsuhashi T, Criado JM. Kinetic analysis of solid-state processes. J Mater Res. 2001;16:1862–71.
Lascano D, Quiles-Carrillo L, Balart R, Boronat T, Montanes N. Kinetic analysis of the curing of a partially biobased epoxy resin using dynamic differential scanning calorimetry. Polymers (Basel). 2019;11(3):391. https://doi.org/10.3390/polym11030391.
Acknowledgements
This research was supported by 2021 Kyungpook National University BK21 FOUR Graduate Innovation Project (International Joint Research Project for Graduate Students). This work was also financially supported by the Program for Leading Talents (no. 2017HA013), the 111 Project (D21027) and the Yunnan Provincial Academician Workstation (YSZJGZZ-2020052).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, S., Yang, L., Park, BD. et al. Thermal cure kinetics of highly branched polyurea resins at different molar ratios. J Therm Anal Calorim 148, 6389–6405 (2023). https://doi.org/10.1007/s10973-023-12160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12160-x