Abstract
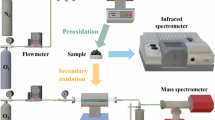
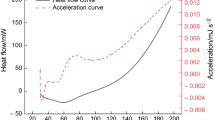
In order to explore the characteristics of spontaneous combustion of Jurassic coal in northern Shaanxi with low metamorphic degree and high coke content, DSC differential scanning calorimeter and diffuse in situ infrared spectroscopy experiment were used to study and analyze the dynamic evolution law and interaction relationship between macroscopic thermal effect and microstructure in the spontaneous combustion process of different coal seams in northern Shaanxi. The results show that there are three characteristic temperature points in the heat flow curve during the spontaneous combustion of Jurassic coal in northern Shaanxi, which can be divided into four stages according to the characteristic temperature points. It was found that the consumption and production of functional groups in Jurassic coal oxidation process were mainly in the absorption bands of aliphatic hydrocarbon, aromatic hydrocarbon and oxygen-containing functional groups, and CH3, CH2, C–H, OH and C=O were the most active key functional groups in the process of coal oxidation. The dynamic correlation degree between heat flux intensity and key active groups is established by using dynamic grey correlation mathematics method. It is concluded that C=O bond has the highest correlation with heat flow intensity during coal oxidation, and has the greatest influence on heat flow intensity. The correlation values of the active groups CH3 and CH2 are second only to the C=O bond, and they are easy to react with oxygen, accompanied by the reaction heat. Although the correlation degree between C–H and OH bond is lower than that of C=O, CH3 and CH2 bond, the correlation degree value is also high, and has certain contribution to the coal reaction heat. These research results have important guiding significance for the development of targeted inhibitors for Jurassic coal spontaneous combustion disaster in northern Shaanxi.









Similar content being viewed by others
References
Ren SJ, Ma T, Zhang YN, Deng J, Xiao Y, Zhai XW, Zhang YT, Song ZY, Wang CP. Sound absorption characteristics of loose bituminous coal porous media with different metamorphic degrees. Fuel. 2023;332: 126091.
Nimaje DS, Tripathy DP. Characterization of some Indian coals to assess their liability to spontaneous combustion. Fuel. 2016;163:139–47.
Avila C, Wu T, Lester E. Petrographic characterization of coals as a tool to detect spontaneous combustion potential. Fuel. 2014;125:173–82.
Deng J, Wang K, Zhang YN, Yang H. Study on the kinetics and reactivity at the ignition temperature of Jurassic coal in North Shaanxi. J Therm Anal Calorim. 2014;118(1):417–23.
Wang K, Han T, Deng J, Zhang YN. Comparison of combustion characteristics and kinetics of Jurassic and Carboniferous-Permian coals in China. Energy. 2022;254: 124315.
Akgün F, Arisoy A. Effect of particle size on the spontaneous heating of a coal stockpile. Combust Flame. 1994;99(1):137–46.
Xiao Y, Li QW, Deng J, Shu CM, Wang W. Experimental study on the corresponding relationship between the index gases and critical temperature for coal spontaneous combustion. J Therm Anal Calorim. 2017;127(1):1009–17.
Obracaj D, Korzec M, Vu TT. The Influence of the Sample Preparation on the Result of Coal Propensity to Spontaneous Combustion in the High-temperature Adiabatic Method. J Polish Mineral Eng Soc. 2021;2(1):65–78.
Jiang XY, Yang SQ, Zhou BZ, Hou ZS, Zhang CS. Effect of gas atmosphere change on radical reaction and indicator gas release during coal oxidation. Fuel. 2022;312: 122960.
Zhang YN, Chen L, Zhao JY, Deng J, Yang H. Evaluation of the spontaneous combustion characteristics of coal of different metamorphic degrees based on a temperature-programmed oil bath experimental system. J Loss Prevent Proc. 2019;60:17–27.
Ren LF, Li QW, Xiao Y, Hao JC, Yi X, Zou L, Li ZB. Critical parameters and risk evaluation index for spontaneous combustion of coal powder in high-temperature environment. Case Stud Therm Eng. 2022;38: 102331.
Li ZB, Wang FS, Wei YQ, Liang R, Gao W, Zhang XF. Thermokinetic analysis of low-rank bituminous coal during low-temperature oxidation: A case study of the Jurassic coal in Shendong coalfield, Ordos Basin, China. Energy 2022;244(Part B):123029.
Wojtacha-Rychter K, Smoliński A. The interaction between coal and multi-component gas mixtures in the process of coal heating at various temperatures: An experimental study. Fuel. 2018;213(9):150–7.
Zhai XW, Ge H, Wang TY, Shu CM, Li J. Effect of water immersion on active functional groups and characteristic temperatures of bituminous coal. Energy. 2020;205: 118076.
Beamish BB, Barakat MA, George JD. An adiabatic testing procedure for determining the self-heating propensity of coal has been evaluated using New Zealand coals. Thermochim Acta. 2000;362:79–87.
Beamish BB, Barakat MA, George JD. Spontaneous-combustion propensity of New Zealand coals under adiabatic conditions. Int J Coal Geol. 2001;45(2):217–24.
Beamish BB, Theiler J. Coal spontaneous combustion: Examples of the self-heating incubation process. Int J Coal Geol. 2019;215: 103297.
Zhang D, Yang XS, Deng J, Wen H, Xiao Y, Jia H. Research on coal spontaneous combustion period based on pure oxygen adiabatic oxidation experiment. Fuel. 2021;288: 119651.
Kong B, Li ZH, Wang EY, Lu W, Chen L, Qi GS. An experimental study for characterization the process of coal oxidation and spontaneous combustion by electromagnetic radiation technique. Process Saf Environ. 2018;119:285–94.
Kong B, Liu Z, Yao QG. Study on the electromagnetic spectrum characteristics of underground coal fire hazardous and the detection criteria of high temperature anomaly area. Environ Earth Sci. 2021;80(3):1–12.
Rathsack P. Analysis of pyrolysis liquids obtained from the slow pyrolysis of a German brown coal by comprehensive gas chromatography mass spectrometry. Fuel. 2017;191:312–21.
Qu J, Deng J, Luo ZM, Xiao Y, Shu C-M. Thermal reaction characteristics and microstructure evolution of aluminium nano-powder in various mixtures of oxygen and nitrogen atmosphere. Process Saf Environ Prot. 2023;170:45–53.
Wang CP, YangY, Tsai YT, Deng J, Shu CM. Spontaneous combustion in six types of coal by using the simultaneous thermal analysis-Fourier transform infrared spectroscopy technique. J Therm Anal Calorim 2016;126(3):1591–1602.
Clemensa AH, Mathesona TW, Rogers DE. Low temperature oxidation studies of dried New Zealand coals. Fuel. 1991;70(2):215–21.
Chen XK, Ma T, Zhai XW, Lei CK. Thermogravimetric and infrared spectroscopic study of bituminous coal spontaneous combustion to analyze combustion reaction kinetics. Thermochim Acta. 2019;676:84–93.
Baysal M, Yürüm A, Yıldız B, Yürüm Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int J Coal Geol. 2016;163:166–76.
Ren LF, Tang H, Xiao Y, Zhang HM, Li QW, Ma T, Inhibiting effects of a proanthocyanidins/sodium polyacrylate composite on the spontaneous combustion of long-flame coal. J Therm Anal Calorim 2022.
Pan JN, Zhu HT, Hou QL. Macromolecular and pore structures of Chinese tectonically deformed coal studied by atomic force microscopy. Fuel. 2015;139:94–101.
Xin HH, Wang DM, Qi XY, Qi GS, Dou GL. Structural characteristics of coal functional groups using quantum chemistry for quantification of infrared spectra. Fuel Process Technol. 2014;118:287–95.
Mo JJ, Xue Y, Liu XQ, Qiu NX, Chu W, Xie HP. Quantum chemical studies on adsorption of CO2 on nitrogen-containing molecular segment models of coal. Surf Sci. 2013;616:85–92.
Painter PC, Snyder RW, Starsinic M, Coleman MM, Kuehn DW, Davis A. Concerning the application of FT-IR to the study of coal: a critical assessment of band assignments and application of spectral analysis programs. Appl Spectrosc. 1981;35(5):475–85.
Ibarra JV, Muñoz E, Moliner R. FTIR study of the evolution of coal structure during the coalification process. Org Geochem. 1996;24(6):725–35.
Yao SP, Zhang K, Jiao K, Hu WX. Evolution of coal structures: FTIR analyses of experimental simulations and naturally matured coals in the Ordos Basin. China Energ Explor Exploit. 2011;29(1):1–19.
Solomon PR, Carangelo RM. FTIR analaysis of coal. 1. techniques and determination of hydroxyl concentrations. Fuel 1982;61:663−669.
Xiong G, Li YS, Jin LJ, Hu HQ. In situ FT-IR spectroscopic studies on thermal decomposition of the weak covalent bonds of brown coal. J Anal Appl Pyrol. 2015;115:262–7.
Niu ZY, Liu GJ, Yin H, Wu D, Zhou CC. Investigation of mechanism and kinetics of non-isothermal low temperature pyrolysis of perhydrous bituminous coal by in-situ FTIR. Fuel. 2016;172:1–10.
Niu ZY, Liu GJ, Yin H, Zhou CC, D. Wu D,Yousaf B, Wang CM. In-situ FTIR study of reaction mechanism and chemical kinetics of a Xundian lignite during non-isothermal low temperature pyrolysis. Energy Convers Manage 2016;124:180–188.
Zhang YL, Wang JF, Xue S, Wu JM, Chang LP, Li ZF. Kinetic study on changes in methyl and methylene groups during low-temperature oxidation of coal via in-situ FTIR. Int J Coal Geol. 2016;154–155:55–164.
Kidena K, Murakami M, Murata S, Nomura M. Low-Temperature Oxidation of Coal−Suggestion of Evaluation Method of Active Methylene Sites. Energ Fuel. 2003;17(4):1043–7.
Tahmasebi A, Yu JL, Han YN, Li XC. A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air. Fuel Process Technol. 2012;101(1):85–93.
Ma T, Chen XK, Zhai XW, Bai YE. Thermogravimetric and infrared spectroscopic study of spontaneous combustion characteristics of different pre-oxidized lignite. RSC Adv. 2019;56(9):32476–89.
Lin XC, Wang CH, Ideta K, Miyawaki J, Nishiyama Y, Wang YG, Yoon S, Mochida I. Insights into the functional group transformation of a Chinese brown coal during slow pyrolysis by combining various experiments. Fuel. 2014;118:257–64.
Misz M, Fabiańska M, Ćmiel S. Organic components in thermally altered coal waste: Preliminary petrographic and geochemical investigations. Int J Coal Geol. 2009;71(4):405–24.
Clemens AH, Matheson TW, Rogers DE. Low temperature oxidation studies of dried New Zealand coals. Fuel. 1991;70(2):215–21.
Zhang YT, Yang CP, Li YQ, Huang Y, Zhang J, Zhang YB, Li QP. Ultrasonic extraction and oxidation characteristics of functional groups during coal spontaneous combustion. Fuel. 2019;242:287–94.
Wang DM, Xin HH, Qi XY, Dou GL, Qi GS, Ma LY. Reaction pathway of coal oxidation at low temperatures: a model of cyclic chain reactions and kinetic characteristics. Combust Flame. 2016;163:447–60.
Fujitsuka H, Ashida R, Kawase M, Miura K. Examination of low-temperature oxidation of low-rank coals, aiming at understanding their self-ignition tendency. Energ Fuel. 2014;28(4):2402–7.
Deng JL. Introduction to Grey system theory. J Grey Syst-uk. 1989;1(1):1–24.
Deng JL. Control problems of grey systems. Syst Control Lett. 1982;1(5):288–94.
Acknowledgements
This work was supported by the Youth Innovation Team of Shaanxi Universities, China (No. 22JP046), the National Natural Science Foundation of China (No. 52204238, No. 51974236, No. 52074215) and the Youth Project of Basic Natural Science Research Program of Shaanxi Province, China (No. 2022JQ-446).
Author information
Authors and Affiliations
Contributions
TM: Conceptualization, Methodology, Writing–original draft preparation, Funding acquisition, S-JR: Writing—review & editing, Methodology, Funding acquisition, X-WZ: Supervision, Funding acquisition, Y-EB: Revision, BS: Methodology, LH: Validation, L-FR: Methodology, X-KC: Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, T., Ren, Sj., Zhai, Xw. et al. Dynamic behavior of oxidative heat release of key active groups for different Jurassic coal seams in northern Shaanxi. J Therm Anal Calorim 148, 4853–4865 (2023). https://doi.org/10.1007/s10973-023-12027-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12027-1