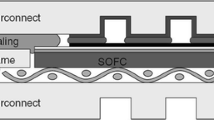
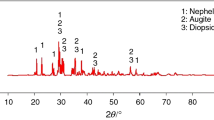
Abstract
In this study, three different mixtures including natural volcanic rock basalt, waste window glass, and Na2CO3 were developed as sealants for using solid oxide fuel cell (SOFC). The mixtures were melted at 1500 °C and cast into the water and graphite mold. The bulk glass parts were used in dilatometer analysis to determine the coefficient of thermal expansion. Glass cast into the water were milled and sized − 53 μm and, after certain pretreatments, coated on Crofer, AISI 409 and 439 stainless steel by dip-coating method. After the coating, heat treatment was performed at 850, 900, and 950 °C in order to ensure permanent bonding and sealing between glass–ceramic sealing and steel substrate. As a result of the heat treatment, the transformation from a glassy structure to a glass–ceramic structure occurred. The interface between glass–ceramic sealing and steel substrate, bonding, and sealing properties were examined by scanning electron microscopy and X-ray diffraction analysis to determine its suitability for SOFC. Furthermore, the activation energies for the precipitation of crystalline phases have been evaluated, and the crystallization mechanisms have been studied by DTA results. The crystallization kinetics was described in terms of the nucleation growth Johnson–Mehl–Avrami model. The results showed that surface crystallization became more effective instead of volume crystallization with increasing Na content.











Similar content being viewed by others
References
Veluswamy GK, Laycock CJ, Shah K, Ball AS, Guwy AJ, Dinsdale RM. Biohythane as an energy feedstock for solid oxide fuel cells. Int J Hydrog Energy. 2019. https://doi.org/10.1016/j.ijhydene.2019.08.256.
Bouraiou A, Necaibia A, Boutasseta N, Mekhilef S, Dabou R, Ziane A, Sahouane N, Attoui I, Mostefaoui M, Touaba O. Status of renewable energy potential and utilization in Algeria. J Clean Prod. 2020. https://doi.org/10.1016/j.jclepro.2019.119011.
Longo S, Cellura M, Guarino F, Brunaccini G, Ferraro M. Life cycle energy and environmental impacts of a solid oxide fuel cell micro-CHP system for residential application. Sci Total Environ. 2019. https://doi.org/10.1016/j.scitotenv.2019.05.368.
Lian J, Zhang Y, Ma C, Yang Y, Chaima E. A review on recent sizing methodologies of hybrid renewable energy systems. Energy Convers Manag. 2019. https://doi.org/10.1016/j.enconman.2019.112027.
Ayawanna J, Kingnoi N, Laorodphan N. Effect of bismuth oxide on crystallization and sealing behavior of barium borosilicate glass sealant for SOFCs. J Non-Crystal Solids. 2019. https://doi.org/10.1016/j.jnoncrysol.2019.01.028.
Mejía Gómez AE, Lamas DG, Leyva AG, Sacanell J. Nanostructured La0.5Ba0.5CoO3 as cathode for solid oxide fuel cells. Ceram Int. 2019. https://doi.org/10.1016/j.ceramint.2019.04.122.
Timurkutluk B, Celik S, Ucar E. Effects of solid loading on joining and thermal cycling performance of glass-ceramic sealing pastes for solid oxide fuel cells. Ceram Int. 2019. https://doi.org/10.1016/j.ceramint.2019.03.207.
Yilmaz S, Ates A, Ercenk E. Crystallization kinetics of basalt-based glass-ceramics for solid oxide fuel cell application. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7414-5.
Ercenk E, Cicekli AE, Yilmaz S. The glass-ceramic sealant materials obtained from basalt for SOFC. J Ceram Pro Res. 2016. https://hdl.handle.net/20.500.12619/69847.
Salman SM, Salama SN, Abo-Mosallam HA. Contribution of some divalent oxides replacing Li2O to crystallization characteristics and properties of magnetic glass–ceramics based on Li2O–Fe2O3–Al2O3–SiO2. Ceram Int. 2016. https://doi.org/10.1016/j.ceramint.2016.02.097.
Zhao L, Wei W, Bai H, Zhang X, Cang D. Synthesis of steel slag ceramics: chemical composition and crystalline phases of raw materials. Int J Miner Metall Mater. 2015. https://doi.org/10.1007/s12613-015-1077-z.
Ghiara G, Piccardo P, Bongiorno V, Geipel C, Spotorno R. Characterization of metallic interconnects extracted from solid oxide fuel cell stacks operated up to 20,000 h in real life conditions: the air side. Energies. 2020. https://doi.org/10.3390/en13246487.
Singhal SC, Kendall K. High temperature SOFC: fundamentals, design and applications. Oxford: Elsevier; 2003.
Wu J, Stebbins JF. Temperature and modifier cation field strength effect on aluminaborosilicate glass network structure. J Non-Crystal Solids. 2013. https://doi.org/10.1016/j.jnoncrysol.2012.11.005.
Morin EI, Wu J, Stebbins JF. Modifier cation (Ba, Ca, La, Y) field strength effects on aluminum and boron coordination in aluminoborosilicate glasses: the role of fictive temperature and boron content. Appl Phys A. 2014. https://doi.org/10.1007/s00339-014-8369-4.
Massera J, Hupa L, Hupa M. Influence of the partial substitution of CaO with MgO on the thermal properties and in vitro reactivity of the bioactive glass S53P4. J Non-Crystal Solids. 2012. https://doi.org/10.1016/j.jnoncrysol.2012.06.032.
Zheng W, Cao H, Zhong J, Qian S, Peng Z, Shen C. CaO–MgO–Al2O3–SiO2 glass-ceramics from lithium porcelain clay tailings for new building materials. J Non-Crystal Solids. 2015. https://doi.org/10.1016/j.jnoncrysol.2014.11.002.
Ji R, Zheng Y, Zou Z, Chen Z, Wei S, Jin X, Zhang M. Utilization of mineral wool waste and waste glass for synthesis of foam glass at low temperature. C Build Mat. 2019. https://doi.org/10.1016/j.conbuildmat.2019.04.226.
NIIR Board of Consultants & Engineers. the complete book an glass-ceramic technology. India: Asia Pasific Business Press; 2017.
Onodera Y, Takimoto Y, Hijiya H, Taniguchi T, Urata S, Inaba S, Fujita S, Obayashi I, Hiraoka Y, Kohara S. Origin of the mixed alkali effect in silicate glass. NPG Asia Mater. 2019. https://doi.org/10.1038/s41427-019-0180-4.
Ito S, Taniguchi T, Ono M, Uemura K. Network and void structure for glasses with a higher resistance to crack formation. J Non-Crystal Solids. 2012. https://doi.org/10.1016/j.jnoncrysol.2012.02.039.
Mcmillan PW. Glass-ceramics. London: Academic Press; 1979.
Sidebottom DL. Connecting glass-forming fragility to network topology. Front Mater. 2019. https://doi.org/10.3389/fmats.2019.00144.
Tan KH, Rahman HA, Taib H. Coating layer and influence of transition metal for ferritic stainless steel interconnector solid oxide fuel cell: A review. Int J Hydrog Energy. 2019. https://doi.org/10.1016/j.ijhydene.2019.06.155.
Timurkutluk B, Toros S, Onbilgin S, Korkmaz HG. Determination of formability characteristics of Crofer 22 APU sheets as interconnector for solid oxide fuel cells. Int J Hydrog Energy. 2018. https://doi.org/10.1016/j.ijhydene.2018.04.243.
Shermer HF. Thermal expansion of binary alkali silicate glasses. J Res Natl Bur Stand. 1956;57:97–101.
Jurado-Egea JR, Owen AE, Bandyopadhyay AK. Electronic conduction in basalt glass and glass-ceramics - correlation with magnetite crystallization. J Mater Sci. 1987. https://doi.org/10.1007/BF01161466.
da Silveira RA, Evaristo LL, Faita FL, Buchner S. Effect of high pressure and high temperature on the Na2O⋅2CaO⋅3SiO2 glass-ceramic’s structural properties. J Non-Crystal Solids. 2021. https://doi.org/10.1016/j.jnoncrysol.2021.121026.
Luo Z, He F, Zhang W, Xiao Y, Xie J, Sun R, Xie M. Effects of fluoride content on structure and properties of steel slag glass-ceramics. Mat Chem Phy. 2020. https://doi.org/10.1016/j.matchemphys.2019.122531.
Khater GA, Yue Y, Abu Safiah MO, Mahmoud MA. Synthesis and characterization of anorthite and magnetite glass-ceramics from basaltic rocks. SILICON. 2019. https://doi.org/10.1007/s12633-018-9991-0.
Cao Y, Xu C, Tian Y, Hou Y. Effect of roasting temperature on phase transformation in co-reduction roasting of nickel slag. Metals. 2020. https://doi.org/10.3390/met10040550.
Petric A, Ling H. Electrical conductivity and thermal expansion of spinels at elevated temperatures. J Am Ceram Soc. 2007. https://doi.org/10.1111/j.1551-2916.2007.01522.x.
Hovis GL, Crelling J, Wattles D, Dreibelbis B, Dennison A, Keohane M, Brennan S. Thermal expansion of nepheline-kalsilite crystalline solutions. Miner Mag. 2003. https://doi.org/10.1180/0026461036730115.
Mysen B, Richet P. Silicate glasses and melts. Amsterdam: Elsevier Book; 2019.
Wachtman JB. Mechanical and thermal properties of ceramics. In: Proceedings of a symposium held at Gaithersburg; 1968.
Kazmina O, Tokareva AY, Vereshchagin VI. Using quartzofeldspathic waste to obtain foamed glass material. Resour Eff Technol. 2016. https://doi.org/10.1016/j.reffit.2016.05.001.
Yang J, Lian J, Bai H, Cui W, Guo Z. Cr2O3 film formed by surface oxidation of stainless steel irradiated by a Ng-YAG pulsed laser. ISIJ Int. 2005. https://doi.org/10.2355/isijinternational.45.730.
Shen YS, Liu J, Guo J, Zhao Y. Mechanisms involved in the roasting of pellets composed of stainless steel slag and sodium hydroxide to extract chromium. ISIJ Int. 2016. https://doi.org/10.2355/isijinternational.ISIJINT-2016-320.
Mittal A, Albertsson GJ, Gupta GS, Seetharaman S, Subramanian S. Some thermodynamic aspects of the oxides of chromium. Metall Mater Trans B. 2014. https://doi.org/10.1007/s11663-014-0027-x.
Liu D, Xue X, Yang H. The effect of chromium on the roasting process of vanadium extraction. Metals. 2016. https://doi.org/10.3390/met6070157.
Li W, Yan X, Aberle AG, Venkataraj S. Effect of sodium diffusion on the properties of CIGS solar absorbers prepared using elemental Se in a two-step process. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-39283-2.
Tiffany Chen HY, Tosoni S, Pacchioni G. A DFT study of the acid–base properties of anatase TiO2 and tetragonal ZrO2 by adsorption of CO and CO2 probe molecules. Surf Sci. 2016. https://doi.org/10.1016/j.susc.2016.02.008.
Karamanov A, Pisciella P, Pelino M. The effect of Cr2O3 as a nucleating agent in iron-rich glass-ceramics. J Eur Ceram Soc. 1999. https://doi.org/10.1016/S0955-2219(99)00047-3.
Ray CS, Huang W, Day DE. Crystallization kinetic of lithia-silica glasses: effect of composition and nucleation agent. J Am Ceram Soc. 1987. https://doi.org/10.1111/j.1151-2916.1987.tb05714.x.
Mahadevan S, Giridhar A, Singh AK. Calorimetric measurements on As–Sb–Se glasses. J Non-Crystal Sol. 1986. https://doi.org/10.1016/S0022-3093(86)80084-9.
Francis AA. Crystallization kinetics of magnetic glass–ceramics prepared by the processing of waste materials. Mat Res Bull. 2006. https://doi.org/10.1016/j.materresbull.2005.11.002.
Balaya P, Sunandana CS. Crystallization studies of 30Li2O:70TeO2 glass. J Non-Crystal Solids. 1993;162–3:253–62.
Author information
Authors and Affiliations
Contributions
EE contributed to conceptualization; SY provided methodology; TY and SY performed formal analysis and investigation; EE and SY performed writing—original draft preparation; EE and SY performed writing—reviewing and editing; ASD and TY provided resources; ASD performed visualization; TY carried out data curation; and SY performed supervision.
Corresponding author
Ethics declarations
Ethical approval
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ercenk, E., Yasar, T., Demirkiran, S. et al. Glass–ceramic sealant with different alkali contents made from natural and waste materials for SOFC. J Therm Anal Calorim 148, 4015–4031 (2023). https://doi.org/10.1007/s10973-023-12007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12007-5