Abstract
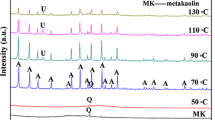
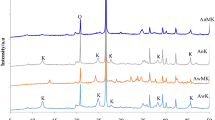
Analcime was prepared by hydrothermal transformation of metakaolin-based geopolymer activated by means of sodium water glass. Gradual transformation of geopolymer through primary and unstable zeolitic phases up to final analcime was studied as a function of hydrothermal treatment duration (6–48 h) and access of water vapour under the selected conditions (165 °C, 0.5 MPa). Composition, microstructure, and thermal stability of the prepared samples were assessed using simultaneous thermogravimetry and differential scanning calorimetry, X-ray diffraction and high-temperature XRD analyses, and scanning electron microscopy. In the case of shorter autoclaving and access of water vapour, the mix of different zeolitic phases was formed: analcime, zeolite P2 gmelinite-Na, and chabazite-Na. From the time of 24 h and autoclaving in the closed moulds, pure cubic analcime was detected. Uniform microstructure of these samples comprised of trapezoid particles with the diameter between 50 and 60 µm. Prolongation of autoclaving time did not lead to the significant change of particle size and their composition. Dehydration of detected zeolites took place through the formation of defect or unknown zeolitic structures before the structural collapse happened.















Similar content being viewed by others
References
Azizi SN, Yousefpour M. Synthesis of zeolites NaA and analcime using rice husk ash as silica source without using organic template. J Mater Sci. 2010;45:5692–7. https://doi.org/10.1007/s10853-010-4637-7.
Sandoval MV, Henao JA, Rios CS, Williams CD, Apperley DC. Synthesis and characterization of zeotype ANA framework by hydrothermal reaction of natural clinker. Fuel. 2009;88:272–81. https://doi.org/10.1016/j.fuel.2008.08.017.
Querol X, Moreno N, Urnana JC, Alastuey A, Hernandez E, Lopez-Soler A, Plana F. Synthesis of zeolites from coal fly ash: an overview. Int J Coal Geol. 2002;50:413–23. https://doi.org/10.1016/S0166-5162(02)00124-6.
Zhang PP, Chen XG, Cheng JP, Ye Y. Preparation of analcime from palygorskite. J. Mater. Sci. Eng. 2010;126:501–4.
Dyer A, Tangkawanit S, Rangsriwatananon K. Exchange diffusion of Cu2+, Ni2+, Pb2+ and Zn2+ into analcime synthesized from perlite. Microporous Mesoporous Mater. 2004;75:273–9. https://doi.org/10.1016/j.micromeso.2004.07.007.
Ma X, Yang J, Ma H, Liu Ch, Zhang P. Synthesis and characterization of analcime using quartz syenite powder by alkali-hydrothermal treatment. Microporous Mesoporous Mater. 2015;201:134–40. https://doi.org/10.1016/j.micromeso.2014.09.019.
Johnson EBG, Arshad SE. Hydrothermally synthesized zeolites based on kaolinite: a review. Appl Clay Sci. 2014;97–98:215–21. https://doi.org/10.1016/j.clay.2014.06.005.
Brough A, Katz A, Bakharev T, Sun G, Kirkpatrick R, Struble L, Young J. Microstructural aspects of zeolite formation in alkali activated cements containing high levels of fly ash. MRS Proc. 1994;370:199–208. https://doi.org/10.1557/PROC-370-197.
Rodríguez ED, Bernal SA, Provis JL, Paya J, Monzo JM, Borrachero MV. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cem Concr Compos. 2013;35:1–11. https://doi.org/10.1016/j.cemconcomp.2012.08.025.
Palomo A, Alonso S, Fernandez-Jimenez A. Alkaline activation of fly ashes: NMR study of the reaction products. J Am Ceram Soc. 2004;87:1141–5. https://doi.org/10.1111/j.1551-2916.2004.01141.x.
Yan H, Xue-Min C, Jin M, Liu LP, Liu XD, Chen JY. The hydrothermal transformation of solid geopolymers into zeolites. Microporous Mesoporous Mater. 2012;161:187–92. https://doi.org/10.1016/j.micromeso.2012.05.039.
Jihong Y. Synthesis of zeolites. In: Čejka J, van Bekkum H, Corma A, Schüth F, editors. Introduction to zeolite science and practice; studies in surface science and catalysis. Amsterdam: Elsevier; 2007. p. 39–103. https://doi.org/10.1016/s0167-2991(07)80791-9.
Alkan M, Hopa C, Yilmaz Z, Güler H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005;86:176–84. https://doi.org/10.1016/j.micromeso.2005.07.008.
Reyes CAR, Williams CD, Alarcón OMC. Synthesis of zeolite LTA from thermally treated kaolinite. Rev Fac Ing Univ Antioq. 2010;53:30–41.
Panagiotopoulou C, Tsivilis S, Kakali G. Application of the Taguchi approach for the composition optimization of alkali activated fly ash binders. Constr Build Mater. 2015;91:17–22. https://doi.org/10.1016/j.conbuildmat.2015.05.005.
Khalid HR, Lee NK, Park SM, Abbas N, Lee HK. Synthesis of geopolymer supported zeolites via robust one-step method and their adsorption potential. J Hazard Mater. 2018;353:522–33. https://doi.org/10.1016/j.jhazmat.2018.04.049.
Provis JL, Lukey GC, van Deventer JSJ. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem Mater. 2005;17:3075–85. https://doi.org/10.1021/cm050230i.
Oh JE, Monteiro PJM, Jun SS, Choi S, Clark SM. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem Concr Res. 2010;40:189–96. https://doi.org/10.1016/j.cemconres.2009.10.010.
Liu ZH, Tang Q, Li CM, He Y, Cui XM. Preparation of NaA zeolite spheres from geopolymer gels using a one-step method in silicone oil. Int J Appl Ceram Technol. 2017;14:982–6. https://doi.org/10.1111/ijac.12708.
Rożek P, Król M, Mozgawa W. Geopolymer–zeolite composites: a review. J Clean Prod. 2019;230:557–79. https://doi.org/10.1016/j.jclepro.2019.05.152.
Lahoti M, Narang P, Tan KH, Yang E-H. Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram Int. 2017;2017:11433–41. https://doi.org/10.1016/j.ceramint.2017.06.006.
Arbel Haddad MA, Ofer-Rozovsky E, Bar-Nes G, Borojovich EJC, Nikolski A, Mogiliansky D, Katz A. Formation of zeolites in metakaolin-based geopolymers and their potential application for Cs immobilization. J Nucl Mater. 2017;493:168–79. https://doi.org/10.1016/j.jnucmat.2017.05.046.
Mazzi F, Galli E. Is each analcime different? Am Mineral. 1978;63:448–60.
Sakizci M. Investigation of thermal and structural properties of natural and ion-exchanged analcime. Anadolu Univ J Sci Technol A Appl Sci Eng. 2016;17:724–34. https://doi.org/10.18038/aubtda.266863.
Todorović M, Radak-Jovanović Z, Gal IJ, Dyer A. The release of tritiated water from synthetic analcime into surrounding water colloids. Surf Sci. 1987;23:345–51. https://doi.org/10.1016/0166-6622(87)80275-5.
Liu J, Fang Y. Study on the disposition of water in fly ash-based geopolymers using ATR-IR. In: 5th international conference on durability of concrete structures, Jun 30–Jul 1, 2016, Shenzhen University, Shenzhen, Guangdong Province, P. R. China. 2016, pp. 163–70. https://doi.org/10.5703/1288284316126.
White CE, Provis JL, Proffen T, van Deventer JSJ. The effects of temperature on the local structure of metakaolin-based geopolymer binder: a neutron pair distribution function investigation. J Am Ceram Soc. 2010;93:3486–92. https://doi.org/10.1111/j.1551-2916.2010.03906.x.
Duxson P, Lukey GC, van Deventer JSJ. Physical evolution of Na-Geopolymer derived from metakaolin up to 1000 °C. J Mater Sci. 2007;42:3044–54. https://doi.org/10.1007/s10853-006-0535-4.
Vereshchagina TA, Kutikhina EA, Solovyov LA, Vereshchagin SN, Mazurova EV, Chernykh YY, Anshits AG. Synthesis and structure of analcime and analcime–zirconia composite derived from coal fly ash cenospheres. Microporous Mesoporous Mater. 2018;258:228–35. https://doi.org/10.1016/j.micromeso.2017.09.011.
Kohoutková M, Kloužková A, Maixner J, Mrázová M. Preparation and characterization of analcime by X-ray and SEM analyses. Ceram Silik. 2007;51:9–14.
Azizi SN, Daghigh AA, Abrishamkar M. Phase transformation of zeolite P to Y and analcime zeolites due to changing the time and temperature. J Spectrosc. 2013. https://doi.org/10.1155/2013/428216.
Tucker MG, Keen DA, Dove MT. A detailed structural characterization of quartz on heating through the α–β phase transition. Mineral Mag. 2001;65:489–507.
Cruciani G. Zeolites upon heating: factors governing their thermal stability and structural changes. J Phys Chem Solids. 2006;67:1973–94. https://doi.org/10.1016/j.jpcs.2006.05.057.
van Reeuwijk LP. The thermal dehydration of natural zeolites. PhD Thesis, Wageningen University, Wageningen; 1974.
Cruciani G, Gualtieri A. Dehydration dynamics of analcime by in situ synchrotron powder diffraction. Am Mineral. 1999;84:112–9. https://doi.org/10.2138/am-1999-1-212.
Baur WH. Self-limiting distorsion by antirotating hinges of flexible but noncollapsible frameworks. J Solid State Chem. 1992;97:243–7. https://doi.org/10.1016/0022-4596(92)90031-P.
Bish DL, Carey JW. Thermal behavior of natural zeolites. Rev Mineral Geochem. 2001;45:403–52. https://doi.org/10.2138/rmg.2001.45.13.
Puntis A, Giampaolo G, Graeme-Barber A. High temperature X-ray diffraction and thermogravimetric analysis of the dehydration of analcime, NaAlSi2O6·H2O. EUG VII Strasb Terra Abstr. 1993;5:497.
Line CMB. The behaviour of water in analcime. PhD Thesis, University of Cambridge, Cambridge UK; 1995.
Line CMB, Putnis A, Putnis C, Giampaolo C. The dehydration kinetics and microtexture of analcime from two parageneses. Am Mineral. 1995;80:268–79. https://doi.org/10.2138/am-1995-3-408.
Line CMB, Dove MT, Knight KS, Winkler B. The low temperature behaviour of analcime. 1: high-resolution neutron powder diffraction. Mineral Mag. 1996;60:499–507. https://doi.org/10.1180/minmag.1996.060.400.11.
Acknowledgements
This work was supported by courtesy of the Slovak Research and Development Agency APVV-15-0631, Slovak Grant Agency VEGA No. 2/0097/17 and by the project: Materials Research Centre at FCH BUT—Sustainability and Development, REG LO1211, with financial support from the National Programme for Sustainability I (Ministry of Education, Youth and Sports).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuzielová, E., Žemlička, M., Másilko, J. et al. Influence of hydrothermal treatment parameters on the phase composition of zeolites. J Therm Anal Calorim 142, 37–50 (2020). https://doi.org/10.1007/s10973-020-09784-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09784-8