Abstract
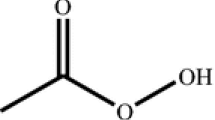
The continuous emergence of new materials and chemical processes has posed great challenge on the hazard assessment capacity, especially the capacity to mimic the reaction progress and to measure the heat released. Although a few thermal hazard screening tools are readily available, a flexible yet cost-effective adiabatic calorimeter that could be applied under extreme conditions is still missing. A house-built high-performance adiabatic calorimeter (HPAC) was developed to solve the problem. We validated the HPAC by comparing its performance with a commercially available Vent Sizing Package 2 in the measurement of H2O2 decomposition and Di-t-butyl peroxide decomposition. We then demonstrated the capability of the HPAC by successfully evaluating the thermal runaway and overpressure hazard of a residual oil hydrotreating reaction under high temperature and high pressure.






Similar content being viewed by others
References
CAS REGISTRY. http://www.cas.org/content/chemical-substances. Accessed 04 Feb 2019.
Iwata Y, Koseki H. Risk evaluation on the basis of pressure rate measured by automatic pressure tracking adiabatic calorimeter. J Hazard Mater. 2008;159(1):35–41.
ARC. http://www.thermalhazardtechnology.com. Accessed 04 Feb 2019.
VSP2. https://www.fauske.com/. Accessed 04 Feb 2019.
Dewar. http://www.chilworth.co.uk. Accessed 04 Feb 2019.
Phitech. http://www.helgroup.com. Accessed 04 Feb 2019.
Askonas CF, Burelbach JP, Leung JC. The versatile VSP2: a tool for adiabatic thermal analysis and vent sizing applications. In: North American thermal analysis society, 28th annual conference, Orlando, 2000.
Li X-R, Koseki H. Study on the early stage of runaway reaction using Dewar vessels. J Loss Prevent Proc. 2005;18(4–6):455–9.
Lin WH, Wu SH, Shiu GY, Shieh SS, Shu CM. Self-accelerating decomposition temperature (SADT) calculation of methyl ethyl ketone peroxide using an adiabatic calorimeter and model. J Therm Anal Calorim. 2009;95(2):645–51.
Wu S-H, Chi J-H, Huang C-C. Thermal hazard analyses and incompatible reaction evaluation of hydrogen peroxide by DSC. J Therm Anal Calorim. 2010;102(2):563–8.
Shu C-M, Yang Y-J. Using VSP2 to separate catalytic and self-decomposition reactions for hydrogen peroxide in the presence of hydrochloric acid. Thermochim Acta. 2002;392–393:259–69.
Liu P, Liu X, Kubota S, Huang P, Wada Y. Thermal oxidation process and characteristic of abietic acid and gum rosin by accelerating rate calorimeter (ARC). J Therm Anal Calorim. 2019;138(1):479–88.
Wei JM, You ML, Chu YC, Shu CM. Evaluation of thermal hazard for lauroyl peroxide by VSP2 and TAM III. J Therm Anal Calorim. 2012;109(3):1237–43.
Jiang HC, Lin WC, Hua M, Pan XH, Shu CM, Jiang JC. Analysis of thermal stability and pyrolysis kinetic of dibutyl phosphate-based ionic liquid through thermogravimetry, gas chromatography/mass spectrometry, and Fourier transform infrared spectrometry. J Therm Anal Calorim. 2019;138(1):489–99.
Sun F, Zhang F, Jin M-P. Vent sizing of cumene hydroperoxide (CHP) system under fire scenario considering emergency flooding measure. J Loss Prevent Proc. 2014;32:230–7.
Kersten RJA, Boers MN, Stork MM. Results of a Round-Robin with di-tertiary-butyl peroxide in various adiabatic equipment for assessment of runaway reaction hazards. J Loss Prevent Proc. 2005;18(3):145–51.
Speight JG. Chapter 4: Hydrotreating and desulfurization. In: Heavy and extra-heavy oil upgrading technologies. Boston: Gulf Professional Publishing; 2013. p. 69–94.
Acknowledgements
The authors acknowledge the support received from the National Key R&D Program of China (2016YFB0301701) and the Chinese State Key Laboratory of Chemical Safety. The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, H., Sun, B., Jiang, J. et al. Measurement of hazardous reactions under extreme conditions with a house-built high-performance adiabatic calorimeter. J Therm Anal Calorim 143, 3817–3823 (2021). https://doi.org/10.1007/s10973-020-09289-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09289-4