Abstract
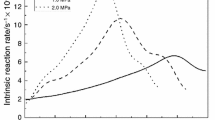
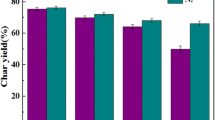
We investigated the release behaviors and reaction reactivities of gaseous products during CO2 gasification of three chars (Guanghui char, Hongshagang char, and Shenmu char) and graphite using thermogravimetric–mass spectrometry. The three chars with different sulfur contents had similar release behaviors. CO was the primary gaseous product, while SO2 was the main gaseous sulfur product. CO forms through the reaction between carbon and CO2. The temperature ranges at which H2 and COS evolved are similar to that of CO, and both H2S and SO2 were released at around 1100 °C. For the same gas, the differences in release behaviors were mainly due to the reactivity of the evolution reactions. Alkali mineral species had a catalytic effect on char gasification, while the increasing degree of graphitization tended to increase gasification reaction resistance. Larger surface area resulted in decreased reaction resistance at the initial stage of the gasification reaction. The most important factor influencing sulfur release reactivity was gasification activity rather than sulfur species in char. Moreover, acidic minerals may accelerate the release of sulfur.














Similar content being viewed by others
References
Society CER. China energy outlook 2030. In: House EMP, editor. Bejing2016.
Kok MV, Yildirim B. Gasification profiles of Thrace region coal under CO2, N2/CO2, and N2/DRY air environments. J Pet Sci Eng. 2019;175:237–45. https://doi.org/10.1016/j.petrol.2018.12.050.
Leeuw KA, Strydom CA, Bunt JR, van Niekerk D. The influence of K2CO3 and KCl on H2 formation during heat treatment of an acid-treated inertinite-rich bituminous coal-char. J Therm Anal Calorim. 2016;126(2):905–12. https://doi.org/10.1007/s10973-016-5597-1.
Jiang Y, Zong P, Tian B, Xu F, Tian Y, Qiao Y, et al. Pyrolysis behaviors and product distribution of Shenmu coal at high heating rate: a study using TG–FTIR and Py-GC/MS. Energy Convers Manag. 2019;179:72–80. https://doi.org/10.1016/j.enconman.2018.10.049.
Wang X, Si J, Tan H, Ma L, Pourkashanian M, Xu T. Nitrogen, sulfur, and chlorine transformations during the pyrolysis of straw. Energy Fuels. 2010;24(9):5215–21. https://doi.org/10.1021/ef1007215.
Xia H, Wei K. Equivalent characteristic spectrum analysis in TG–MS system. Thermochim Acta. 2015;602:15–21. https://doi.org/10.1016/j.tca.2014.12.019.
Lin Y, Huang Q, Wei K, Xia H. Quantitative study on adsorption and regeneration characteristics of activated coke using equivalent characteristic spectrum analysis. Ind Eng Chem Res. 2019;58(12):5080–6. https://doi.org/10.1021/acs.iecr.9b00309.
Lin Y, Zheng M, Ye C, Power IM. Thermogravimetric analysis–mass spectrometry (TGA–MS) of hydromagnesite from Dujiali Lake in Tibet, China. J Therm Anal Calorim. 2018;133(3):1429–37. https://doi.org/10.1007/s10973-018-7197-8.
Justh N, Berke B, László K, Szilágyi IM. Thermal analysis of the improved Hummers’ synthesis of graphene oxide. J Therm Anal Calorim. 2018;131(3):2267–72. https://doi.org/10.1007/s10973-017-6697-2.
Zhao B, Jin J, Li S, Liu D, Zhang R, Yang H. Co-pyrolysis characteristics of sludge mixed with Zhundong coal and sulphur contaminant release regularity. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08300-x.
Huang QWK, Xia H. Investigations in the recrystallization of evolved gases from pyrolysis process of melamine. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08338-x.
Qian H, Kai W, Hongde X. A novel perspective of dolomite decomposition: elementary reactions analysis by thermogravimetric mass spectrometry. Thermochim Acta. 2019;676:47–51. https://doi.org/10.1016/j.tca.2019.03.042.
Fan D, Zhu Z, Na Y, Lu Q. Thermogravimetric analysis of gasification reactivity of coal chars with steam and CO2 at moderate temperatures. J Therm Anal Calorim. 2013;113(2):599–607. https://doi.org/10.1007/s10973-012-2838-9.
Song HJ, Liu GR, Zhang JZ, Wu JH. Pyrolysis characteristics and kinetics of low rank coals by TG–FTIR method. Fuel Process Technol. 2017;156:454–60. https://doi.org/10.1016/j.fuproc.2016.10.008.
Fidalgo B, Menendez JÁ. Carbon materials as catalysts for decomposition and CO2 reforming of methane: a review. Chin J Catal. 2011;32(1–2):207–16. https://doi.org/10.1016/S1872-2067(10)60166-0.
Gee SY, Chang SK, Park HL. Oxidation processes in CAS and the binding-energy of S2p in CASO4. Phys Status Solidi A Appl Res. 1991;125(2):K89–91. https://doi.org/10.1002/pssa.2211250241.
Zhang LJ, Li ZH, Yang YL, Zhou YB, Li JH, Si LL, et al. Research on the composition and distribution of organic sulfur in coal. Molecules. 2016;21(5):13. https://doi.org/10.3390/molecules21050630.
Tang H, Xu M, Hu H, Yang F, Yang Y, Liu H, et al. In-situ removal of sulfur from high sulfur solid waste during molten salt pyrolysis. Fuel. 2018;231:489–94. https://doi.org/10.1016/j.fuel.2018.05.123.
Zhang Y, Wang M, Qin Z, Yang Y, Fu C, Feng L, et al. Effect of the interactions between volatiles and char on sulfur transformation during brown coal upgrade by pyrolysis. Fuel. 2013;103:915–22. https://doi.org/10.1016/j.fuel.2012.09.061.
Blasing M, Muller M. Release of alkali metal, sulphur, and chlorine species from high temperature gasification of high- and low-rank coals. Fuel Process Technol. 2013;106:289–94. https://doi.org/10.1016/j.fuproc.2012.08.010.
Wang X, Guo H, Liu F, Hua R, Wang M. Effects of CO2 on sulfur removal and its release behavior during coal pyrolysis. Fuel. 2016;165:484–9. https://doi.org/10.1016/j.fuel.2015.10.047.
Qiu K, Anthony EJ, Jia L. Oxidation of sulfided limestone under the conditions of pressurized fluidized bed combustion. Fuel. 2001;80(4):549–58. https://doi.org/10.1016/s0016-2361(00)00128-9.
Guo L, Zuo H, Wang Y, Zhao J. Thermal behavior and kinetic study on the pyrolysis of lean coal blends with thermally dissolved coal. J Therm Anal Calorim. 2019;136(2):903–12. https://doi.org/10.1007/s10973-018-7719-4.
Wako FM, Reshad AS, Goud VV. Thermal degradation kinetics study and thermal cracking of waste cooking oil for biofuel production. J Therm Anal Calorim. 2018;131(3):2157–65. https://doi.org/10.1007/s10973-017-6760-z.
Fang W. Study on reaction kinetic of steam-coal chars gasification with TGA. J China Coal Soc. 2004;29(03):350–3.
Chunxia X, Zhengang X, Xuepeng B, Weiguo D. Characteristic study on the co-gasification of coal chars with CO2 and steam. J China Coal Soc. 2009;34(07):952–6.
Zhao H, Cao Y, Orndorff W, Pan W-P. Gasification characteristics of coal char under CO2 atmosphere. J Therm Anal Calorim. 2014;116(3):1267–72. https://doi.org/10.1007/s10973-013-3627-9.
Hou A, Wang Z, Song W, Lin W. Thermogravimetric analysis on gasification reactivity of Hailar lignite influence of inherent mineral matters and external ash. J Therm Anal Calorim. 2012;109(1):337–43. https://doi.org/10.1007/s10973-011-1712-5.
Duman G, Uddin MA, Yanik J. The effect of char properties on gasification reactivity. Fuel Process Technol. 2014;118:75–81. https://doi.org/10.1016/j.fuproc.2013.08.006.
Zhan X, Jia J, Zhou Z, Wang F. Influence of blending methods on the co-gasification reactivity of petroleum coke and lignite. Energy Convers Manag. 2011;52(4):1810–4. https://doi.org/10.1016/j.enconman.2010.11.009.
Bai Y, Lv P, Yang X, Gao M, Zhu S, Yan L, et al. Gasification of coal char in H2O/CO2 atmospheres: evolution of surface morphology and pore structure. Fuel. 2018;218:236–46. https://doi.org/10.1016/j.fuel.2017.11.105.
Anthony EJ, Jia L, Qiu K. CaS Oxidation by Reaction with CO2 and H2O. Energy Fuels. 2003;17(2):363–8. https://doi.org/10.1021/ef020124s.
Zhou Y, Li S, Li P, Kong Y, Zhao H. Sulfur-retention characteristics of different coal mixtures. J Power Eng. 2012;32(3):217–21. https://doi.org/10.1007/s11783-011-0280-z.
Attar A. Chemistry, thermodynamics and kinetics of reactions of sulfur in coal-gas reactions: review. Fuel. 1978;57(4):201–12. https://doi.org/10.1016/0016-2361(78)90117-5.
Acknowledgements
This work was financially supported by National Key Research and Development Program of China (No. 2017YFB0602302) and Beijing Municipal Science and Technology Project (No. Z181100005118006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xian, S., Zhang, H., Chai, Z. et al. Release characteristics of gaseous products during CO2 gasification of char. J Therm Anal Calorim 140, 177–187 (2020). https://doi.org/10.1007/s10973-019-08704-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08704-9