Abstract
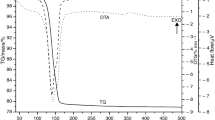
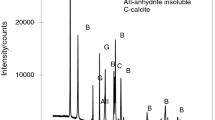
The paper presents the results of studies of the effect of water-soluble impurities on the thermal dehydration of phosphogypsum in a self-generated atmosphere, which are obtained by thermogravimetry and differential scanning calorimetry. For comparison, the results of the thermal dehydration of gypsum are given. The information about the change in activation energy depending on the extent of the dehydration and the effect of temperature on the mechanism of the dehydration is obtained by the use of integral isoconversional and model-fitting methods. It has been established that water-soluble impurities have a significant influence on the kinetics of the dehydration. It has been shown that in the thermal dehydration of gypsum an abnormal change in the reaction rate and activation energy is observed. In the dehydration of phosphogypsum, this phenomenon is not observed. This is due to the impact of the water-soluble impurities on the recrystallization of an intermediate amorphous phase during the thermal decomposition.











Similar content being viewed by others
References
Andreev MV, Brodskiy AA, Zabeleshenskiy YA, Zorina EA, Klenitskiy AI, Kochetkov VN, Rodin VI, Evenchik SD. Chapter 9. Economy of production of phosphorus-containing fertilizers. In: Andreev MV, Brodskiy AA, Zabeleshenskiy YA, Zorina EA, Klenitskiy AI, Kochetkov VN, Rodin VI, Evenchik SD, editors. Technology of phosphorus and complex fertilizers. Moscow: Khimia; 1987. p. 397–433.
Ivanitskiy VV, Klassen PV, Stonis SN, Evenchik SD, Yakovleva ME. Chapter 1. Phosphogypsum. In: Ivanitskiy VV, Klassen PV, Stonis SN, Evenchik SD, Yakovleva ME, editors. Phosphogypsum and its use. Moscow: Khimia; 1990. p. 8–47.
Heffner P, Prud’momme M. Fertilizer Outlook 2016–2020, 84th IFA Annual Conference, Moscow. 30 May–1 June 2016. http://www.fertilizer.org/imis20/images/Library_Downloads/2016_IFa_Moscow_Summary.pdf.
Tayibi H, Choura M, Lopez FA, Alguacil FJ, Lopez-Delgado A. Environmental impact and management of phosphogypsum. J Environ Manag. 2009;90:2377–86. https://doi.org/10.1016/j.jenvman.2009.03.007.
Hentati O, Abrantes N, Caetano AL, Bouguerra S, Goncalves F, Römbke J, Pereira R. Phosphogypsum as a soil fertilizer: ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J Hazard Mater. 2015;294:80–9. https://doi.org/10.1016/j.jhazmat.2015.03.034.
Kuryatnyk T, Angulski da Luz C, Ambroise J, Pera J. Valorization of phosphogypsum as hydraulic binder. J Hazard Mater. 2008;160:681–7. https://doi.org/10.1016/j.jhazmat.2008.03.014.
Cuadri AA, Navarro FJ, García-Morales M, Bolívar JP. Valorization of phosphogypsum waste as asphaltic bitumen modifier. J Hazard Mater. 2014;279:11–6. https://doi.org/10.1016/j.jhazmat.2014.06.058.
Crangle, Jr. RD. Gypsum (advance release), 2014 Minerals Yearbook. U.S. Geological Survey, November 2016. https://minerals.usgs.gov/minerals/pubs/commodity/gypsum/myb1-2014-gypsu.pdf.
Ball BC, Norwood LS. Studies in the system calcium sulphate–water. Part III. Kinetics of dehydration of α-calcium sulphate hemihydrate. J Chem Soc A Inorg Phys Theor. 1970. https://doi.org/10.1039/J19700001476.
Christensen AN, Olesen M, Cerenius Y, Jensen TR. Formation and transformation of five different phases in the CaSO4–H2O system: crystal structure of the subhydrate β-CaSO4·0.5H2O and soluble anhydrite CaSO4. Chem Mater. 2008;20:2124–32. https://doi.org/10.1021/cm7027542.
Ball BC, Norwood LS. Studies in the system calcium sulphate–water. Part I. Kinetics of dehydration of calcium sulphate dihydrate. J Chem Soc A Inorg Phys Theor. 1969. https://doi.org/10.1039/J19690001633.
Abriel W, Reisdorf K, Pannetier J. Dehydration reactions of gypsum: a neutron and X-ray diffraction study. J Solid State Chem. 1990;85:23–30. https://doi.org/10.1016/S0022-4596(05)80055-6.
Lopez FA, Tayibi H, Garcia-Diaz I, Alguacil FJ. Thermal dehydration kinetics of phosphogypsum. Mater Constr. 2015. https://doi.org/10.3989/mc.2015.07214.
Lou W, Guan B, Wu Z. Dehydration behavior of FGD gypsum by simultaneous TG and DSC analysis. J Therm Anal Calorim. 2011;104:661–9. https://doi.org/10.1007/s10973-010-1100-6.
Paulik F, Paulik J, Arnold M. Thermal decomposition of gypsum. Thermochim Acta. 1992;200:195–204. https://doi.org/10.1016/0040-6031(92)85115-C.
Isa K, Okuno H. Thermal decomposition of calcium sulfate dehydrate under self-generated atmosphere. Bull Chem Soc Jpn. 1982;55:3733–7. https://doi.org/10.1246/bcsj.55.3733.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92. https://doi.org/10.1002/app.1961.070051506.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6:639–42. https://doi.org/10.1002/app.1962.070062406.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6. https://doi.org/10.1246/bcsj.38.1881.
Flynn JH, Wall LA. A quick, direct method for the determination of action energy from thermogravimetric data. J Polym Sci Part C Polym Lett. 1966;4:323–8. https://doi.org/10.1002/pol.1966.110040504.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Sunol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23. https://doi.org/10.1016/j.tca.2014.05.036.
Hammam MAS, Abdel-Rahim MA, Hafiz MM, Abu-Sehly AA. New combination of non-isothermal kinetics-revealing methods. Study of Se90Te10 powders with heterogenius particle sizes and shapes. J Therm Anal Calorim. 2017;128:1391–405. https://doi.org/10.1007/s10973-017-6086-x.
Vyazovkin S, Wight CA. Isothermal and non-isothermal reaction kinetics in solids: in search of ways toward consensus. J Phys Chem A. 1997;101:8279–84. https://doi.org/10.1021/jp971889h.
Ghaderi F, Nemati M, Siahi-Shadbsd MR, Valizadeh H, Monajjemzadeh F. Evaluation of activation energy conformity derived from model free non-isothermal predictions and Arrhenius isothermal results. The case of hydrochlorothiazide–lactose reaction. J Therm Anal Calorim. 2017;130:1417–27. https://doi.org/10.1007/s10973-017-6279-3.
Andreev MV, Brodskiy AA, Zabeleshenskiy YA, Zorina EA, Klenitskiy AI, Kochetkov VN, Rodin VI, Evenchik SD. Chapter 2. Production of phosphoric acid. In: Andreev MV, Brodskiy AA, Zabeleshenskiy YA, Zorina EA, Klenitskiy AI, Kochetkov VN, Rodin VI, Evenchik SD, editors. Technology of phosphorus and complex fertilizers. Moscow: Khimia; 1987. p. 61–116.
Follner S, Wolter A, Helming K, Silber C, Bartels H, Follner H. On the real structure of gypsum crystals. Cryst Res Technol. 2002;37:207–18. https://doi.org/10.1002/1521-4079(200202)37:2/3<207::AID-CRAT207>3.0.CO;2-L.
Lü P, Fei D, Dang Y. Effects of calcium monohydrogenphosphate on the morphology of calcium sulfate whisker by hydrothermal synthesis. Can J Chem Eng. 2014;92:1709–13. https://doi.org/10.1002/cjce.22037.
Kruger A, Focke WW, Kwela Z, Fowles R. Effect of ionic impurities on the crystallization of gypsum in wet-process phosphoric acid. Ind Eng Chem Res. 2001;40:1364–9. https://doi.org/10.1021/ie000478b.
Hasson D, Addai-Mensah J, Metcalfe J. Filterability of gypsum crystallized in phosphoric acid solution in the presence of ionic impurities. Ind Eng Chem Res. 1990;29:867–75. https://doi.org/10.1021/ie00101a023.
Kruger A, Focke WW, Kwela Z. Effect of ferrous and ferric iron impurities on the crystallization of gypsum and sludge formation in wet-process phosphoric acid. Chem Eng Commun. 2002;189:684–94. https://doi.org/10.1080/00986440211740.
Jun L, Jian Hua W, Yun Xiang Z. Effects of the impurities on the habit of gypsum in wet-process phosphoric acid. Ind Eng Chem Res. 1997;36:2657–61. https://doi.org/10.1021/ie960422a.
Palou M, Kuzielova E, Zemlicka M, Novotny R, Masilko J. The effect of metakaoline upon the formation of ettringite in metakaoline–lime–gypsum ternary systems. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6885-0.
Jo D, Leonardo RS, Cartledge FK, Mendoza Reales OA, Toledo Filho RD. Gypsum content determination in Portland cements by thermogravimetry. J Therm Anal Calorim. 2016;123:1053–62. https://doi.org/10.1007/s10973-015-5078-y.
Antar K, Jemal M. A thermogravimetric study into the effects of additives and water vapor on the reduction of gypsum and Tunisian phosphogypsum with graphite or coke in a nitrogen atmosphere. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6871-6.
Lyakhov NZ, Boldyrev VV. Kinetics and mechanism of the dehydration of crystal hydrates. Rus Chem Rev. 1972;41:919–28. https://doi.org/10.1070/RC1972v041n11ABEH002103.
Galwey AK. Structure and order in thermal dehydrations of crystalline solids. Thermochim Acta. 2000;355:181–238. https://doi.org/10.1016/S0040-6031(00)00448-2.
Ball MC, Urea RG. Studies in the system calcium sulphate–water Part II The kinetics of dehydration of β-CaSO4·1/2H2O. J Chem Soc A Inorg Phys Theor. 1970. https://doi.org/10.1039/j19700000528.
Dollimore D. The influence of the environmental on the thermal decomposition of oxysalts. J Therm Anal Calorim. 1977;11:185–200. https://doi.org/10.1007/BF01909956.
Makatun VN, Shchegrov LN. State of water in inorganic crystal hydrates and the characteristic features of their dehydration. Russ Chem Rev. 1972;41:905–18. https://doi.org/10.1070/RC1972v041n11ABEH002102.
Mandal PK, Mandal TK. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O). Cem Concr Res. 2002;32:313–6. https://doi.org/10.1016/S0008-8846(01)00675-5.
Okazaki S, Yamazaki M. The character of acid sites on the gypsum surface. Bull Chem Soc Jpn. 1981;54:436–40. https://doi.org/10.1246/bcsj.54.436.
Peng Y, Zhu Z, Braatz RD, Myerson AS. Gypsum crystallization during phosphoric acid production: modeling and experiments using the mixed–solvent–electrolyte thermodynamic model. Ind Eng Chem Res. 2015;54:7914–24. https://doi.org/10.1021/acs.iecr.5b01763.
Kurteva OI, Brutskus EB. On the solubility of calcium sulphate in a mixture of acids H3PO4 + H2SO4 and H3PO4 + H2SiF6. Zh Prikl Khim. 1999;31:1714–22.
Sestak J. Chapter 8. Mechanism and kinetics of heterogeneous non-catalytic reactions. In: Sestak J, editor. Theory of thermal analysis: physicochemical properties of solid inorganic substances. Moscow: Mir; 1987. p. 186–222.
Strydom CA, Hudson-Lamb DL, Potgieter JH, Dagg E. The thermal dehydration of synthetic gypsum. Thermochim Acta. 1995;269–270:631–8. https://doi.org/10.1016/0040-6031(95)02521-9.
Kontogeorgos DA, Founti MA. Gypsum board dehydration kinetics at autogenous water vapour partial pressure. Thermochim Acta. 2012;545:141–7. https://doi.org/10.1016/j.tca.2012.07.009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorbovskiy, K.G., Ryashko, A.I., Kazakov, A.I. et al. The influence of water-soluble impurities on thermal dehydration kinetics of phosphogypsum in self-generated atmosphere. J Therm Anal Calorim 133, 1549–1562 (2018). https://doi.org/10.1007/s10973-018-7272-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7272-1