Abstract
Porous microspheres of glycidyl methacrylate(GMA) cross-linked with trimethylolpropane trimethacrylate (TRIM) were prepared with toluene as porogen by suspension–emulsion polymerization. In order to obtain adsorbents bearing functional groups, the porous methacrylate network was modified by subsequent reaction with pyrrolidone. The thermal behavior of the obtained material was studied using TG and DSC. It was found that the process of modification considerably changed the textural and thermal properties of the polymers.
Similar content being viewed by others
Introduction
Porous polymers possess a number of distinguishing properties like highly developed internal structure, hydrophobic/hydrophilic character, and the presence of various functional groups on the surface. These features make them very attractive from scientific and industrial point of view. Consequently, porous polymers are subject of many scientific investigations and have attracted the attention of producers.
They are used as effective materials for many separation processes and various kinds of sorbents [1–9]. They can be obtained from numerous types of monomers as well as by modification of copolymers that contain reactive groups [10–18]. One of the convenient routes to incorporate new functional group into polymer matrix is ring-opening reaction of oxirane ring with required agent. Widespread practice is reaction of epoxy group with amines [19–22].
This process leads not only to the introduction of the active pendant group to the network but also to the changes in the textural and thermal properties of the newly obtained materials.
Recently, we have described the synthesis and some properties of porous microspheres of glycidyl methacrylate (GMA) cross-linked with trimethylolpropane trimethacrylate (TRIM) modified with pyrrolidone [23].
It was of interest to investigate in detail, how the process of modification influences the thermal resistance of the newly formed copolymers. To achieve this goal, a set of ten copolymers was synthesized. The thermal properties of the parent and modified copolymers were evaluated by the means of TG and DSC. Additionally, the textural characterization was carried out on the basis of the low-temperature nitrogen adsorption on the studied copolymers.
Experimental
Chemicals
2,3-Epoxypropyl methacrylate (GMA) and TRIM (Sigma Aldrich, Steinheim, Germany) were washed with 5 % aqueous sodium hydroxide in order to remove inhibitors. Pyrrolidone bis(2-ethylhexyl) sulfosuccinate sodium salt (DAC,BP) and α,α′-azoisobutyronitrile (AIBN), purchased from Fluka AG (Buchs, Switzerland), were used without purification. Toluene, n-dodecane, acetone, and methanol (reagent grade) were from POCh (Gliwice, Poland).
Preparation of the GMA–TRIM microspheres
Copolymerization was performed in an aqueous suspension medium. In a typical experiment, 195 mL of distilled water and 2.2 g of bis(2-ethylhexyl)sulfosuccinate sodium salt were stirred for 2 h at 80 °C in order to dissolve the surfactant. Then the solution containing 15 g of monomers (GMA and TRIM), and 0.2 g of α,α′-azoisobutyronitrile dissolved in 22.5 mL of toluene was prepared and added while stirring to the aqueous medium. Molar ratios of GMA to TRIM were changed from 1:1 to 5:1. Copolymerization was performed for 20 h at 80 °C. Porous beads formed in this process were filtered off, and an extensive cleaning procedure was applied in order to remove the diluent unreacted monomers and physically adsorbed stabilizer. The cleaning process was as follows: the microspheres were separated from the aqueous phase by filtration of the polymerization mixture by 5-μm filter papers. The microspheres were first washed with water, and the polymeric aggregates were removed by sieving. The microspheres were dispersed in water, and the dispersion was sonificated for 0.5 h in an ultrasonic bath. Next, the water phase was removed, and the microspheres were resuspended in methyl alcohol. This dispersion was sonificated for 1 h. Methyl alcohol was removed, and the microspheres were transferred into toluene and were kept there by stirring about 0.5 h. Then, the toluene was removed, and microspheres were stirred with methyl alcohol for about 0.5 h. Methyl alcohol was removed, and the microspheres were washed with distilled water, filtered, and dried in vacuum oven at 65° for 48 h.
Modification of the epoxy groups
The epoxy groups present in the copolymer were modified during the reaction with pyrrolidone. The procedure was as follows: in a 250 cm3 round-bottomed two-necked flask equipped with a mechanical stirrer and a thermometer, 10 g of selected beads was placed together with the excess of pyrrolidone and left for 24 h to swell. Then the whole content was heated at 150 °C for 8 h. The obtained modified beads were washed with distilled water, filtered off, and cleaned as described above.
Methods of analysis
Textural characterization of the copolymers was carried out by the low-temperature nitrogen adsorption–desorption method. Nitrogen adsorption–desorption isotherms were obtained at the liquid nitrogen temperature using a volumetric adsorption analyzer ASAP 2405 (Micromeritics Inc., USA). The measurements of the porous structure of the copolymers were preceded by outgassing of the samples at 140 °C for 2 h. The specific surface area of the investigated samples was calculated by the Brunauer–Emmet–Teller (BET) method for the adsorption data in the range of a relative pressure p/p o 0.05–0.25. The total pore volume was estimated from a single point adsorption at a relative pressure of 0.985. The pore size was obtained from the desorption branch of the isotherm using the Barrett–Joyner–Halenda (BJH) procedure.
The surface of the obtained beads was also examined using an atomic LEO 1430 VP numerical scanning electron microscope (Germany) with a countershaft and an energy dispersive X-ray detector.
The thermal properties of the synthesized composites were evaluated on the basis of TG and DSC measurements performed using the STA449, F1 Jupiter analyzer from Netzsch (Günzbung, Germany). The procedure was as follows: about 10 mg of the sample was placed in the TG pan and heated in helium or in air atmosphere at a rate of 10 Kmin−1 up to 1,000 °C with the sample mass about 10 mg. The initial decomposition temperature (IDT), T 20%, T 50% of mass loss, and final decomposition temperature (FDT) were determined.
Results and discussion
Porous copolymers of poly(TRIM–GMA) used in this study were synthesized through suspension–emulsion copolymerization in the form of regular microspheres (Fig. 1).
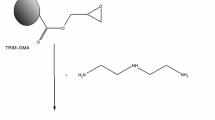
During the synthesis, TRIM served as a cross-linker and was responsible for the mechanical and thermal properties of the resulting polymeric matrix. GMA provided reactive epoxy groups that were modified by subsequent reaction with pyrrolidone (Fig. 2).
The molar ratio of the functional monomer to the cross-linker was increased from 1:1(TRIM–GMA1, TRIM–GMA1 + P) to 1:5 (TRIM–GMA5, TRIM–GMA5 + P). As a result, a set of ten various copolymers was obtained. They differ considerably in terms of parameters of porous structure as well as thermal resistance. The properties of the obtained copolymers are determined by the molar ratio of monomers and the degree of incorporation of pyrrolidone units into polymer matrix.
With the increasing amount of GMA in the polymerization mixture, decreases in the values of porous surface area and pore volume were observed. At the same time, an increase in the pore diameter changes was noticed. This process can be explained on the basis of the compatibility of the polymer network with the porogen expressed by the solubility parameters (δ). For the studied system, the solubility parameters are as follows: 18.20 (MPa)1/2 for TRIM, 19.50 (MPa)1/2 for GMA, and 18.201/2 for toluene. With the increase in GMA content in the poly(TRIM–GMA) copolymers, the difference between the solubility parameters of the polymer network and the porogen increases, and the compatibility of the polymer network with toluene decreases. This phenomenon pulls the trigger earlier phase separation. As a result, large microglobules and large pores are formed, and the porosity is lower (Table 1).
The value of specific surface area decreases from 333 m2g−1 (TRIM–GMA1) to 86 m2g−1 (TRIM–GMA5). The same pattern is observed for the pore volume. The increase in GMA amount in the copolymer network also led to shifting the maximum of the PSD toward a larger pore size.
Modification of the TRIM–GMA copolymers with pyrrolidone results in considerable decreases in the values of specific surface area, pore volume, and pore diameter. This process also considerably changes the thermal properties of the new materials. Tables 2 and 3 contain parameters evaluated from TG and DTG curves of the parent and modified copolymers determined in helium (Figs. 3–6). From these data, one can see that increasing the molar ratio of GMA to TRIM from 1:1 to 5:1 slightly changes the thermal resistance of the copolymers. The IDT decreases from 219 °C for TRIM–GMA1 to 203 °C for TRIM–GMA5. More spectacular differences can be seen while comparing parent and modified copolymers. After modification, the IDT temperature determined in helium is about 60 °C higher for the whole series of copolymers. The differences are even more visible for T 20% and T 50% temperatures. The analysis of DTG curves provides another piece of information. In the case of parent TRIM–GMA copolymers, two separate peaks are visible. The first maximum can be attributed to the decomposition of epoxy group and the second to the process of degradation ester bounds. After the reaction of the epoxy ring with pyrrolidone (P), the DTG curves show only one peak with maximum from 453 °C for TRIM–GMA1 + P to 399 °C for TRIM–GMA4 + P.The same pattern is observed in the case of analysis conducted in air (Figs. 7–10). Along with increasing the molar ratio of GMA to TRIM, the initial decomposition temperature decreases from 232 °C for TRIM–GMA1 copolymer to 225 °C for TRIM–GMA5 (Table 4). The decomposition process proceeded into two steps (Fig. 7). The process of modification epoxy groups with pyrrolidone enhances the thermal resistance of the adsorbents. All of the modified copolymers indicate higher IDT comparing with their initial counterparts (Table 5). The differences in thermal behavior between parent and functionalized copolymers were also noticed during DSC measurements (Figs. 11, 12).
Conclusions
Porous copolymers of glycidyl methacrylate cross-linked with trimethylolpropane trimethacrylate were synthesized in the form of microspheres. In the next step, the reactive epoxy groups were modified by subsequent reaction with pyrrolidone. This process led to significant changes in the textural and thermal properties of the functionalized copolymers.
Modification of the TRIM–GMA copolymers with pyrrolidone results in considerable decreases in the values of specific surface area, pore volume, and pore diameter. What is interesting is that thermal stability of the polymer increases with the introduction of the functional groups. The course of TG, DTG, and DSC curves of parent copolymer is at variance with the functionalized ones.
Abbreviations
- δ :
-
Solubility parameter/(MPa)1/2
- D BJH :
-
Pore diameter/Å
- FDT:
-
Temperature of final decomposition/°C
- IDT:
-
Initial decomposition temperature/°C
- P:
-
Pyrrolidone
- S BET :
-
Specific surface area/m2g−1
- T 1max :
-
Temperature of the first maximum rate of mass loss/°C
- T 2max :
-
Temperature of the second maximum rate of mass loss/°C
- T 20% :
-
Temperature of 20 % mass loss/°C
- T 50% :
-
Temperature of 50 % mass loss/°C
- TRIM–GMA1:
-
The parent copolymer, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:1
- TRIM–GMA2:
-
The parent copolymer, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:2
- TRIM–GMA3:
-
The parent copolymer, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:3
- TRIM–GMA4:
-
The parent copolymer, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:4
- TRIM–GMA5:
-
The parent copolymer, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:5
- TRIM–GMA1 + P:
-
The copolymer modified with pyrrolidone, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:1
- TRIM–GMA2 + P:
-
The copolymer modified with pyrrolidone, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:2
- TRIM–GMA3 + P:
-
The copolymer modified with pyrrolidone, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:3
- TRIM–GMA4 + P:
-
The copolymer modified with pyrrolidone, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:4
- TRIM–GMA5 + P:
-
The copolymer modified with pyrrolidone, molar ratio of trimethylolpropane trimethacrylate to glycidyl methacrylate equal 1:5
- V :
-
Pore volume/cm3g−1
References
Jaćkowska M, Bocian S, Gawdzik B, Grochowicz M, Buszewski B. Influence of chemical modification on the porous structure of polymeric adsorbents. Mat Phys. 2011;130:644–50.
Maciejewska M, Gawdzik J. Preparation and characterization of sorption properties of porous microspheres of 1-vinyl-2-pyrrolidone-divinylbenzene. J Liq Chrom Rel Tech. 2008;31:950–61.
Bielicka-Daszkiewicz K, Voelkel A, Szejner M, Osypiuk J. Extraction properties of new polymeric sorbents in SPE/GC analysis of phenol and hydroquinone from water samples. Chemosphere. 2006;62(6):890–8.
Maciejewska M. Characterization of macroporous 1-vinyl-2-pyrrolidone copolymers obtained by suspension polymerization. J Appl Polym Sci. 2012;124:568–75.
Maciejewska M, Osypiuk-Tomasik J. Studies of sorption properties of porous copolymers of 1-vinyl-2-pyrrolidone. J Therm Anal Calorim. 2013;111:1595–601.
Gokmen MT, Du Prez FE. Porous polymer particles-a comprehensive guide to synthesis, characterization, functionalization and applications. Prog Polym Sci. 2012;37:365–405.
Maciejewska M, Osypiuk-Tomasik J. Sorption on porous copolymers of 1-vinyl-2-pyrrolidone-divinylbenzene. J Therm Anal Calorim. 2013;114:749–55.
Grochowicz M, Gawdzik B, Jaćkowska M, Buszewski B. Thermal characterization of polymeric anion exchangers with a dendrimeric structure. J Therm Anal Calorim. 2013;114:955–61.
Maciejewska M. Influence of the filler on thermal properties of porous VP-TRIM copolymers. J Therm Anal Calorim. doi:10.1007/s10973-014-4118-3.
Grochowicz M. Investigation of the thermal behavior of 4-vinylpyridine–trimethylolpropane trimethacrylate copolymeric microspheres. J Therm Anal Calorim. doi:10.1007/s10973-014-4066-y.
Maciejewska M, Szajnecki Ł, Gawdzik B. Investigation of the surface area and polarity of porous copolymers of maleic anhydride and divinylbenzene. J Appl Polym Sci. 2012;125:300–7.
Gawdzik B, Maciejewska M. Synthesis of isobutyl maleate-divinylbenzene microspheres by different techniques of heterogeneous polymerizations. J Appl Polym Sci. 2004;91:2008–15.
Maciejewska M, Gawdzik B, Grochowicz M. Characterization of the methacrylate copolymers with the use of inverse gas chromatography. Pol J Chem. 2008;82:235–40.
Maciejewska M, Osypiuk J, Gawdzik B. Preparation and characterization of chromatographic properties of ethylene glycol dimethacrylate-divinylbenzene polymeric microspheres. J Polym Sci Part A: Polym Chem. 2005;43:3049–58.
Zaleski R, Kierys A, Grochowicz M, Dziadosz M, Goworek J. Synthesis and characterization of nanostructural polymer-silica composite: positron annihilation lifetime spectroscopy study. J Colloid Interf Sci. 2011;358:268–76.
Maciejewska M, Osypiuk-Tomasik J. TG/DSC studies of modified 1-vinyl-2-pyrrolidone-divinylbenzene copolymers. J Therm Anal Calorim. 2013;113:343–50.
Trochimczuk AW, Kolarz N. Synthesis and chelating properties of resins with methylthiourea, guanylthiourea and dithiocarbamate groups. Europ Polym J. 2000;36:2359–63.
Deryło-Marczewska A, Goworek J, Kusak R, Zgrajka W. Sorption properties of porous melamine – formaldehyde resins. App Sufr Sci. 2002;195:117–25.
Qiu W, Zhang K, Liu J, Koros W, Sun Q, Derng Y. Macroporous polymeric sorbents with high selectivity for separation of phenols. Polymer. 2010;51:3793–800.
Worzakowska M. The influence of tertiary aromatic amines on the BPO initiated cure of unsaturated epoxy polyesters with styrene studied by non-isothermal DSC. J Therm Anal Calorim. 2011;105:987–94.
Podkościelna B, Maciejewska M, Bartnicki A. Studies on synthesis and physicochemical properties of new bis[4-(2-hydroxy-3-methacryloyloxypropoxy) phenyl]sulfide terpolymers. J Appl Polym Sci. 2012;123:59–65.
Drelinkiewicz A, Knapika A, Stanucha W, Sobczak J, Bukowska A, Bukowski W. Diamine functionalized gel-type resin as a support for palladium catalysts: preparation, characterization and catalytic properties in hydrogenation of alkynes. React Funct Polym. 2008;68:1652–64.
Maciejewska M. Kołodyńska D. Synthesis and characterization of porous microspheres bearing pyrrolidone units Mat Chem Phys. doi:10.1016/j.matchemphys.2014.09.026.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Maciejewska, M. Characterization of thermal properties of porous microspheres bearing pyrrolidone units. J Therm Anal Calorim 119, 1147–1155 (2015). https://doi.org/10.1007/s10973-014-4250-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4250-0