Abstract
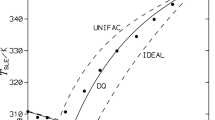
Solid–liquid equilibria for three binary mixtures of N-(2-acetoxyethyl)-p-nitroaniline (1) + 2-nitrodiphenylamine (2), N-(2-acetoxyethyl)-p-nitroaniline (1) + ethyl centralite (2) and N-(2-acetoxyethyl)-p-nitroaniline (1) + methyl centralite (2) have been determined experimentally using differential scanning calorimeter (DSC). Simple eutectic behaviours for these systems have been observed. The experimental results have been correlated by means of NRTL and UNIQUAC equations. The root-mean-square deviations of the solubility temperatures for all measured data vary from 0.61 to 3.32 K and depend on the particular model used. The best solubility correlation has been obtained with the UNIQUAC model.










Similar content being viewed by others
References
Ritter H, Braun S, Kaiser M, Becher C. Stabilizer degradation in propellants: identification of two isomeric forms of 2-nitro-N-nitroso-N-ethylaniline. Propellant Explos Pyrotech. 2008;33:203–8.
Lussier LS, Berger E, Gapnon H. Study of daughter products of Akardite-II. Propellant Explos Pyrotech. 2006;31:253–62.
Bohn MA, Volk F. Aging behavior of propellants investigated by heat generation, stabilizer consumption, and molar mass degradation. Propellant Explos Pyrotech. 1992;17:171–8.
Vogelsanger B. Chemical stability, compatibility and shelf life of explosives. Chimia. 2004;58:401–8.
Trache D, Mazroua A, Khimeche K. Determination of chemical and mechanical properties of propellants during ageing, vol 83. In: Proc. 42nd international annual conference of ICT, Germany; 2011. pp 1–10.
Lindblom T. Reaction in stabilizer and between stabilizer and nitrocellulose in propellants. Propellant Explos Pyrotech. 2002;27:197–208.
Bohn MA. Kinetic modelling of the concentrations of the stabilizer DPA and some of its consecutive products as function of time and temperature. J Therm Anal Calorim. 2001;65:103–20.
Trache D, Khimeche K. Study on the influence of ageing on chemical and mechanical properties of N,N′-dimethyl-N,N′-diphenylcarbamide stabilized propellants. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-012-2320-8.
Zayed MA, Mohamed AA, Hassan MAM. Stability studies of double-base propellants with centralite and malonanilide stabilizers using MO calculations in comparison to thermal studies. J Hazard Mater. 2010;179:453–61.
Boers MN, de Klerk WPO. Lifetime prediction of EC, DPA, akardite II and MNA stabilized triple base propellants, comparison of heat generation rate and stabilizer consumption. Propellant Explos Pyrotech. 2005;30:356–62.
Mekki A, Khimeche K, Dahmani A. Measurement and prediction of (solid + liquid) equilibria of gun powder’s and propellant’s stabilizers mixtures. J Chem Thermodyn. 2010;42:1050–5.
Khimeche K, Dahmani A. Determination by DSC of solid-liquid diagrams for polyaromatic-4,4′Diaminodiphenylmethane binary systems. J Therm Anal Calorim. 2006;84:47–52.
Khimeche K, Dahmani A. Measurement and prediction of (solid + liquid) equilibria of (alkanediamine + biphenyl) mixtures. J Chem Thermodyn. 2006;38:1192–8.
Khimeche K, Boumrah Y, Benziane M, Dahmani A. Solid-liquid equilibria and purity determination for binary n-alkane + naphthalene systems. Thermochim Acta. 2006;444:165–71.
Wittig R, Constantinescu D, Gmehling J. Binary solid–liquid equilibria of organic systems containing ε-caprolactone. J Chem Eng Data. 2001;46:1490–3.
Farahani BV, Rajabi FH, Hosseindoust B, Zenooz N. DSC study of solid-liquid equilibria for energetic binary mixtures of methylnitramine with 2,4-dinitro-2,4-diazapentane and 2,4-dinitro-2,4-diazahexane. J Phase Equilib Diffus. 2010;31:536–41.
Gibson JD. Stabilizers for cross-linked composite modified double base propellants. US Patent 5387285, 2/7/1995.
Chevallier V, Petitjean D, Meray VR, Dirand M. Correlations between the crystalline long c-parameter and the number of carbon atoms of pure n-alkanes. Polymer. 1999;40:5953–6.
Chen YP, Tang M, Kuo JC. Solid–liquid equilibria for binary mixtures of N-phenylacetamide with 4-aminoacetophenone, 3-hydroxyacetophenone and 4-hydroxyacetophenone. Fluid Phase Equilib. 2005;232:182–8.
Mackay D, Shiu WY, Ma K, Lee SC. Handbook of physical-chemical properties and environmental fate for organic chemicals. 2nd ed. Boca Raton: Taylor & Francis; 2006.
Meyer R, Köler J, Homburg A. Explosives. 6th ed. Weinheim: Wiley-VCH; 2007.
Baum EJ. Chemical property estimation, theory and application. Boca Raton: CRC Press LLC; 2000.
Quinchon J, Tranchant J. Les poudres, propergols et explosifs. Tome 2. Edition Lavoisier; 1984.
Lide DR, Baysinger G, Berger LI, Goldberg RN, Haynes WM. CRC handbook of chemistry and physics. 89th ed. Boca Raton: Taylor & Francis, CRC Press; 2009.
Jr Acree WE. Thermodynamic properties of organic compounds. Enthalpy of fusion and melting point temperature compilation. Thermochim Acta. 1991;189:37–40.
Witschonke CR. Freezing point and purity data for some organic compounds. Anal Chem. 1954;26:562–4.
Matsuoka M, Ozawa R. determination of solid-liquid phase equilibria of binary organic systems by differential scanning calorimetry. J Cryst Growth. 1989;96:596–604.
Huang CC. ChenYP, Measurements and model prediction of the solid–liquid equilibria of organic binary mixtures. Chem Eng Sci. 2000;55:3175–85.
Domanska U, Gonzalez JA. Solid-liquid equilibria for systems containing long chain 1-alkanols. II. Experimental data for 1-dodecanol, 1-tetradecanol, 1-hexadecanol, 1-octadecanol or 1-eicosanol + CCl4 or + cyclohexane mixtures. Characterization in terms of DISQUAC. Fluid Phase Equilib. 1996;123:167–87.
Rai RN, Rai US. Solid-liquid equilibrium and thermochemical properties of organic eutectic in a monotectic system. Thermochim Acta. 2000;363:23–8.
Praunitz JM, Lichtenthaler RN, Azevedo EG. Molecular thermodynamics of fluid phase equilibrium. 2nd ed. New Jersey: Prentice-Hall, Englewood Cliff; 1986.
Coutinho JAP, Andersen SI, Stenby EH. Solid-liquid equilibrium of n-alkanes using the Chain Delta Lattice Parameter model. Fluid Phase Equilib. 1996;117:138–45.
Domanska U, Groves JFR, McLaughlin E. Solid-liquid phase equilibria of binary and ternary mixtures of benzene and polynuclear aromatic compounds. J Chem Eng Data. 1993;38:88–94.
Pan C, Radosz M. Modeling of solid–liquid equilibria in naphthalene, normal-alkane and polyethylene solutions. Fluid Phase Equilib. 1999;155:57–73.
Reddi RSB, Satuluri VSAK, Rai RN. Solid–liquid equilibrium, thermal and physicochemical studies of organic eutectics. J Therm Anal Calorim. 2012;107:183–8.
Iddaoudi A, Selhaoui N, Ait Amar M, Mahdouk K, Aharoune A, Bouirden L. Thermodynamic description of the Lu–Pb binary system. J Therm Anal Calorim. 2011; doi:10.1007/s10973-011-1881-2.
Reddi RSB, Satuluri VSAK, Rai US, Rai RN. Thermal, physicochemical and microstructural studies of binary organic eutectic systems. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1478-9.
Rai US, Rai RN. Physical chemistry of organic eutectics. J Therm Anal Calorim. 1998;53:883–93.
Marinescu DC, Pincu E, Meltzer V. Thermal analysis of binary liquid crystals eutectic system cholesteril p-phenoxi phenyl carbamate–cholesteril p-biphenyl carbamate. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1980-0.
Narendra K, Srinivasu C, Kalpana C, Narayanamurthy P. Excess thermo dynamical parameters of binary mixtures of toluene and mesitylene with anisaldehyde using ultrasonic technique at different temperatures. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1521-x.
Mukhopadhyay M, Sahasranaman K. Computation of multicomponent liquid–liquid equilibrium data for aromatics extraction systems. Ind Eng Chem, Process Des Dev. 1982;21:632–40.
Nelder JA, Mead R. A simplex method for function minimization. Comput J. 1965;7:308–13.
Hofman T, Nagata J. Determination of association constants for alcohols based on ethers as homomorphs. Fluid Phase Equilib. 1986;25:113–28.
Monfort JP, Rojas MDL. A study of simplified molecular models in phase equilibrium prediction. Fluid Phase Equilib. 1978;2:181–98.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trache, D., Khimeche, K., Benziane, M. et al. Solid–liquid phase equilibria for binary mixtures of propellant’s stabilizers. J Therm Anal Calorim 112, 215–222 (2013). https://doi.org/10.1007/s10973-012-2539-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2539-4