Abstract
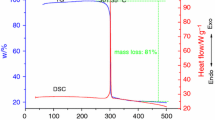
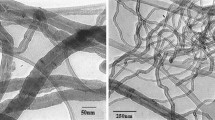
Multi-walled carbon nanotubes (MWCNTs) have remarkable properties. However, their thermal stability characteristics, which may represent potential hazards during the production or utilization stage, concern unsafe or unknown properties researches. Our aim was to analyze the thermokinetic parameters of different heating rates by differential scanning calorimetry (DSC) and thermogravimetric analyzer (TG), and then to compare thermal decomposition energy parameters under various conditions by well-known kinetic equations. MWCNTs were acidified via nitric acid (HNO3) in various concentrations from 3 to 15 N and were characterized by means of Fourier transform infrared (FTIR) spectrometry. For original and modified MWCNTs, we further identified the thermal degradation characteristics of the functional group by TG-FTIR. Finally, we established an effective and prompt procedure for receiving information on thermal decomposition characteristics and reaction hazard of MWCNTs that could be applied as an inherently safer design during normal or upset operation.






Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor (min−1)
- dT/dt :
-
Self-heating rate (°C min−1)
- E a :
-
Activation energy (kJ mol−1)
- H 0 :
-
Initial heat flow (kJ mol−1)
- H T :
-
Heat flow at temperature T (kJ mol−1)
- k 0 :
-
Reaction rate constant (s−1 M1−n)
- m :
-
Mass of reactant (g)
- n :
-
Reaction order (dimensionless)
- R :
-
Universal gas constant (J mol−1 K−1)
- T :
-
Temperature (°C)
- t :
-
Time (min)
- T 0 :
-
Initial exothermic temperature (°C)
- T m :
-
Temperature to maximum of weight loss percentage (°C)
- T p :
-
Peak temperature (°C)
- V :
-
Volume of reactant (L)
- α:
-
Fractional conversion (dimensionless)
- β:
-
Heating rate (°C min−1)
- ρ:
-
Density of reactant (g L−1)
- ΔH :
-
Heat of reaction (kJ mol−1)
- ΔH 0 :
-
Total peak area of DSC curve (kJ mol−1)
- ΔH d :
-
Heat of decomposition (kJ mol−1)
- ΔH T :
-
Heat of decomposition via DSC trial (kJ mol−1)
References
Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–8.
Lu C, Chung YL, Chang KF. Adsorption of trihalomethanes from water with carbon nanotubes. Water Res. 2005;39:1183–9.
Pritchard DK. Literature review–explosion hazards associated with nanopowders. USA: Crown; 2004.
Jin J, Song M, Pan F. A DSC study of effect of carbon nanotubes on crystallization behaviour of poly (ethylene oxide). Thermochim Acta. 2007;456:25–31.
Chervin S, Bodman GT. Mechanism and kinetics of decomposition from isothermal DSC data: development and application. Process Saf Prog. 1997;16:94–100.
Wang YW, Shu CM, Duh YS, Kao CS. Thermal runaway hazards of cumene hydroperoxide with contaminants. Ind Eng Chem Res. 2001;40:1125–32.
Shu CM, Yang YJ. Using VSP2 to separate catalytic and self-decomposition reactions for hydrogen peroxide in the presence of hydrochloric acid, temperatures and oxygen concentrations. Thermochim Acta. 2002;392:257–69.
Hsieh YC, Chou YC, Lin CP, Hsieh TF, Shu CM. Thermal analysis of multi-walled carbon nanotubes by Kissinger’s corrected kinetic equation. Aerosol Air Qual Res. 2010;10:212–8.
Chang CW, Chou YC, Tseng JM, Liu MY, Shu CM. Thermal hazard evaluation of carbon nanotubes with sulfuric acid by DSC. J Therm Anal Calorim. 2008;95(2):639–43.
Peng JJ, Wu SH, Hou HY, Lin CP, Shu CM. Thermal hazards evaluation of cumene hydroperoxide mixed with its derivatives. J Therm Anal Calorim. 2009;96:783–7.
Hou HY, Duh YS, Lee W, Shu CM. Hazard evaluation for redox system of cumene hydroperoxide mixed with inorganic alkaline solutions. J Therm Anal Calorim. 2009;95:541–5.
Paradise M, Goswami T. Carbon nanotubes—production and industrial applications. Mater Des. 2007;28:1477–89.
Mettler Toledo. STARe Thermal Analysis. Sweden; 2005.
Perkin Elmer. Pyris 1 Getting Started Guide. UK; 2006.
Perkin Elmer. SPECTRUM 100 Series Getting Started Guide. UK; 2006.
MWCNTs 2040 COA. Conyuan Biochemical Technology Co. Ltd., Taipei, Taiwan, ROC. http://www.cbt.com.tw.
Berber S, Kwon YK, Tománek D. Unusually high thermal conductivity of carbon nanotubes. Phys Rev Lett. 2000;84(20):4613–6.
Kim P, Shi L, Majumdar A, McEuen PL. Thermal transport measurements of individual multiwalled nanotubes. Phys Rev Lett. 2001;87(21):225502-1–4.
Xie H, Cai A, Wang X. Thermal diffusivity and conductivity of multiwalled carbon nanotube arrays. Phys Lett A. 2007;369:120–3.
Çengel YA, Boles MA. Thermodynamics: an engineering approach. 6th ed. New York: McGraw-Hill Inc.; 2007.
Qi X, Boggs S. Thermal and mechanical properties of EPR and XLPE cable compounds. IEEE Electr Insul Mag. 2006;22(3):19–24.
Jung YS, Jeon DY. Surface structure and field emission property of carbon nanotubes grown by radio-frequency plasma-enhanced chemical vapor deposition. Appl Surf Sci. 2002;193:129–37.
Acknowledgements
The authors are indebted to the donors of the National Science Council of Taiwan under the contract no. NSC 97-2622-E-224-002-CC3 for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chou, YC., Hsieh, TF., Hsieh, YC. et al. Comparisons of MWCNTs and acidified process by HNO3 on thermal stability by DSC and TG-FTIR. J Therm Anal Calorim 102, 641–646 (2010). https://doi.org/10.1007/s10973-010-1017-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1017-0