Abstract
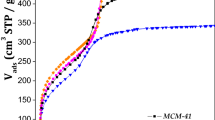
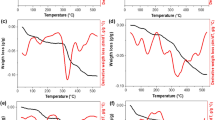
Compositions based on oxides and containing 5–20% w/w of ammonium molybdophosphate have been synthesized by means of different routes. Prepared samples have been studied using nitrogen adsorption–desorption, XRD, DTA–TG, and FTIR spectroscopy. Keggin structure is retained at incorporation of ammonium molybdophosphate into siliceous framework or its deposition on oxide surface and duration following calcinations up to 500 °C. Compositions possess porous structure from micromesoporous to mesomacroporous depending on the preparation method.











Similar content being viewed by others
References
Pope MT. Heteropoly and isopoly oxometallates. Berlin: Springer; 1983.
Pope MT, Müller A. Polyoxometalate chemistry: an old field with new dimensions in several disciplines. Angew Chem Int Ed Engl. 1991;30:34–48.
Okuhara T, Mizuno N, Misono M. Catalytic chemistry of heteropoly compounds. Adv Catal. 1996;41:113–252.
Moffat JB. The surface and catalytic properties of heteropoly oxometalates, fundamental and applied catalysis. New York: Kluwer Academic/Plenum Publishers; 2001.
Cavani F, Mezzogori R, Pigamo A, Trifiro F, Etienne E. Main aspect of the selective oxidation of isobutene to methacrylic acid catalyzed by Keggin-type polyoxometallates. Catal Today. 2001;71:97–110.
Yamaze T. Photo- and electrochromism of polyoxometalates and related materials. Chem Rev. 1998;98:307–26.
Zhang TR, Feng W, Lu R, Bao CY, Li TJ, Zhao YY, Yao JN. Preparation of photochromic sol-gel composite films containing dodecaphosphotungstic acid. Mater Chem Phys. 2002;78:380–4.
Kormali P, Troupis A, Triantis T, Hiskia A, Papaconstantinou E. Photocatalysis by polyoxometallates and TiO2: a comparative study. Catal Today. 2007;124:149–55.
María D, Hernández-Alonso A, Fernando Fresno B, Silvia Suárez A, Juan M. Coronado development of alternative photocatalysts to TiO2: challenges and opportunities. Energy Environ Sci. 2009;2:1231–57.
Sydorchuk V, Zazhigalov V, Khalameida S, Skubiszewska-Zięba J, Charmas B, Leboda R. Deposition of tungsten heteropolycompounds on activated silica surface. Colloids Surf A. 2009;341:53–9.
Tarlani A, Abedini M, Nemati A, Khabaz M, Amini MM. Immobilization of Keggin and Preyssler tungsten heteropolyacids on various functionalized silica. J Colloid Interface Sci. 2006;303:32–8.
Mrowiec-Bialon J, Turek W, Jarz˛ebski AB. Preparation of highly active heteropolyacid-silica composite catalysts using sol–gel method. React Kinet Catal Lett. 2002;76:213–9.
Fuchs V, Mendez L, Blanco M, Pizzio L. Mesoporous titania directly modified with tungstophosphoric acid: synthesis, characterization and catalytic evaluation. Appl Catal A. 2009;358:73–8.
Alcoutlabi M, McKenna GB. Effect of confinement on material behaviour at the nanometer size scale. J Phys Condens Matter. 2005;17:R461–524.
Burt MC, Dave BC. Externaly dynamic confinement effect in organosilica sol-gels. J Am Chem Soc. 2006;128:11750–1.
Amphlett CB. Inorganic ion exchangers. New York: Elsevier; 1964.
Clearfield A. Inorganic ion exchanger materials. Boca Raton: CRC Press; 1982.
Smit J. Insoluble heterolyacid salts. In: Qureshi M, Varshney KG, editors. Inorganic ion exchangers in chemical analysis. Boston: CRC Press; 1991. p. 68–9.
Caletka R, Konečnŷ C. Adsorption properties of ammonium molybdophosphate supported in pores of silica gel. Radiochim Radioanal Lett. 1972;12:325–9.
Doležal J, Stejskal J, Tympl M, Kouřĭm V. Improved inorganic ion-exchangers. II Ammonium molybdophosphate–silica gel system. J Radioanal Chem. 1974;21:381–7.
Terada K, Hayakawa H, Sawada K, Kiba T. Silica gel as a support for inorganic ion-exchangers for the determination of cesium-137 in natural waters. Talanta. 1970;17:955–63.
Tranter TJ, Aloy AS, Sapozhnikova NV, Knecht DA, Todd TA. Porous crystalline silica (gubka) as a inorganic support matrix for novel sorbent. Mat Res Soc Symp. 2002;713: JJ11.68.1–7.
Satyannarayana J, Murthy GS, Sassidhar P. Adsorption studies of cesium on a new inorganic exchanger ammonium molybdophosphate–alumina (AMP–Al2O3). J Radioanal Nucl Chem. 1999;242:11–6.
Matsuda A, Daiko Y, Ishida T, Tadanaga K, Tatsumisago M. Characterization of proton-conducyive SiO2–H3PMo12O40 composites prepared by mechanochemical treatment. Solid State Ion. 2007;178:709–12.
Berezovska IS, Yanishpolskii VV, Tertykh VA. Synthesis of mesoporous silicas inside large pores of inorganic matrix. J Therm Anal Calorim. 2008;94:649–53.
Sidorchuk VV, Tertykh VA, Klimenko VP, Ragulya AV. Formation and some properties of barium titanate embedded into porous matrices. J Therm Anal Calorim. DOI 10.1007?s 10973-010-0814-9.
Heinike G. Tribochemistry. Berlin: Academie Verlag; 1980.
Popa A, Sasca V, Stefanescu M, Kiš EE, Marinković-Nedučin R. The influence of the nature and textural properties of different supports on the thermal behavior of Keggin type heteropolyacids. J Serb Chem Soc. 2006;71:235–49.
Iler RK. Chemistry of silica. New York: Wiley; 1979.
Sydorchuk V, Khalameida S, Zazhigalov V, Skubiszewska-Zięba J, Leboda R, WieczorekCiurowa K. Influence of mechanochemical activation in various media on structure of porous and non-porous silicas. Appl Surf Sci. (in press).
Sasca V, Ştefãnescu M, Popa A. Studies on the non-isothermal decomposition of H3PMo12O40·xH2O and H4PVMo11O40·yH2O. J Therm Anal Calorim. 1999;56:569–78.
Sasca V, Stefanescu M, Popa A. Thermal behavior of the polyoxometalates derived from H3PMo12O40 and H4PVMo11O40. J Therm Anal Calorim. 2003;72:311–22.
Rocchiccioli-Deltcheff C, Amirouche M, Herve G, Fournier M, Che M, Tatibouet JM. Structure and catalytic properties of silica-supported polyoxomolybdates: II. Thermal behavior of unsupported and silica-supported 12-molybdosilicic acid catalysts from IR and catalytic reactivity studies. J Catal. 1990;126:591–9.
Bridgeman AJ. Density functional study of the vibrational frequencies of a Keggin-heteropolyanions. Chem Phys. 2003;287:55–69.
Acknowledgements
This study was supported by International Visegrad Fund (Contract No. 50910373).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sydorchuk, V., Khalameida, S., Skubiszewska-Zięba, J. et al. Synthesis and structure of AMP/oxide support. J Therm Anal Calorim 103, 257–265 (2011). https://doi.org/10.1007/s10973-010-0967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0967-6