Abstract
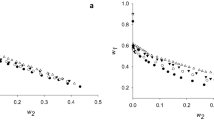
The displacement adsorption enthalpies (ΔH) of denatured α-Amylase (by 1.8 mol L−1 GuHCl) adsorbed onto a moderately hydrophobic surface (PEG-600, the end-group of polyethylene glycol) from solutions (x mol L−1 (NH4)2SO4, 0.05 mol L−1 KH2PO4, pH 7.0) at 298 K are determined by microcalorimeter. Further, entropies (ΔS), Gibbs free energies (ΔG) and the fractions of ΔH, ΔS, and ΔG for net adsorption of protein and net desorption of water are calculated in combination with adsorption isotherms of α-Amylase based on the stoichiometric displacement theory for adsorption (SDT-A) and its thermodynamics. It is found that the displacement adsorptions of denatured α-Amylase onto PEG-600 surface are exothermic and enthalpy driven processes, and the processes of protein adsorption are accompanied with the hydration by which hydrogen bond form between the adsorbed protein molecules favor formation of β-sheet and β-turn structures. The Fourier transformation infrared spectroscopy (FTIR) analysis shows that the contents of ordered secondary structures of adsorbed α-Amylase increase with surface coverages and salt concentrations increment.


Similar content being viewed by others
References
Kim KS, Kim S, Yang HJ, Kwon DY. Changes of glycinin conformation due to pH, heat and salt determined by DSC and CD. Int J Food Sci Technol. 2004;39:385–93.
Lin FY, Chen CS, Chen WY. Yamamoto Sc. Microcalorimetric studies of the interaction mechanisms between proteins and Q-sepharose at pH near the isoelectric point (pI) effects of NaCl concentration, pH value, and temperature. J Chromatogr A. 2001;912:281–9.
Bai SF, Dong AC. Effects of immobilization onto aluminum hydroxide particles on the thermally induced conformational behavior of three model proteins. Int J Biol Macromol. 2009;45:80–5.
Geng XP, Wu YN, Wang BH, Zhang HF, Geng XD, Xing JW. Microcalorimetric determination of displacement adsorption enthalpies of protein refolding on a moderately hydrophobic surface at 308 K. J Therm Anal Calorim. 2006;85:601–8.
Huang HM, Lin FY, Chen WY, Ruaan RC. Isothermal titration microcalorimetric studies of the effect of temperature on hydrophobic interaction between proteins and hydrophobic adsorbents. J Colloid Interface Sci. 2000;229:600–6.
Bian LJ, Feng WK, Geng XD. Prediction of retention for protein in hydrophobic interaction chromatography. J Chromatogr (in chinese). 1991;9:267–70.
Tsai YS, Lin FY, Chen WY, Lin CC. Isothermal titration microcalorimetric studies of the effect of salt concentrations in the interaction between proteins and hydrophobic adsorbents. Colloids Surf A. 2002;197:111–8.
Geng XP, Wu YN, Song JR, Geng XD, Xing JW, Lei ZM. Effect of salt concentrations on the displacement adsorption enthalpies of denatured protein folding at a moderately hydrophobic surface. J Therm Anal Calorim. 2006;85:593–600.
Geng XP, Zheng MR, Wang BH, Lei ZM, Geng XD. Fractions of thermodynamic functions for native lysozyme adsorption onto moderately hydrophobic surface. J Therm Anal Calorim. 2008;93:503–8.
Geng XP, Gao H, Wang BH, Liu AL, Feng XY. Thermodynamic analysis of denatured lysozyme folded on moderately hydrophobic surface at 298 K. J Therm Anal Calorim. 2009;95:345–52.
Geng XD. Stoichiometric displacement theory and its application. Beijing: Science Press; 2004. (in chinese).
Geng XP, Han TS, Cao C. Adsorption enthalpy and desorption enthalpy during displacement of adsorbate to solvent in a liquid–solid system. J Therm Anal. 1995;45:157–65.
Geng XP. Study on the fractions of thermodynamic function changes for both adsorption and desorption from a liquid–solid system. Thermochim Acta. 1998;308:131–8.
Geng XP, Zhang HF, Wang BH, Geng XD, Xing JW. Calorimetric determination of enthalpies of lysozyme folding at a liquid–solid interface. J Therm Anal Calorim. 2005;82:193–9.
Byler DM, Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986;25:469–87.
Byler DM, Brouillette JN, Susi H. Quantitative studies of protein structure by FT-IR spectra deconvolution and curve-fitting. Spectroscopy. 1986;1:29–32.
Schwinté P, Voegel JC, Picart C, Haikel Y, Schaaf P, Szalontai B. Stabilizing effects of various polyelectrolyte multilayer films on the structure of adsorbed/embedded fibrinogen molecules: an ATR-FTIR study. J Phys Chem B. 2001;105:11906–16.
Yan LF, Sun ZR. Protein molecule structure. Beijing: Tsinghua University Press; 1999. (in chinese).
Brandes N, Welzel PB, Werner C, Kroth LW. Adsorption-induced conformational changes of proteins onto ceramic particles: differential scanning calorimetry and FTIR analysis. J Colloid Interface Sci. 2006;299:56–9.
Lu Y, Zhang WW, Wang GK. Progress in study of secondary structure of denaturized protein by FTIR. Spectrosc Spectr Anal (in chinese). 2008;28:88–93.
Lei ZM, Geng XP, Dai L, Geng XD. DSC and FTIR study of adsorbed lysozyme on hydrophobic surface. Spectrosc Spectr Anal (in chinese). 2008;28:2058–61.
Sane SU, Cramer SM, Przybycien TM. Protein structure perturbations on chromatographic surfaces. J Chromatogr A. 1999;849:149–59.
Norde W, Favier JP. Structure of adsorbed and desorbed proteins. Colloids Surf. 1992;64:87–93.
Acknowledgements
We thank the National Natural Science Foundation of China for sponsoring the project (Grant No. 20673080).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, X.Y., Geng, X.P., Peng, J.J. et al. Thermodynamic investigation of effect of salt concentrations on denatured α-Amylase adsorbed onto a moderately hydrophobic surface. J Therm Anal Calorim 102, 799–807 (2010). https://doi.org/10.1007/s10973-009-0611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0611-5