Summary
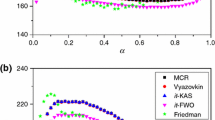
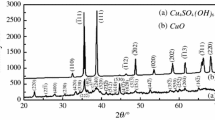
The paper contains an analysis of the used of Diefallah's composite integral method of kinetic parameters evaluation. It is shown that the application of this method should be preceded by the application of an isoconversional method through which the dependence of the activation energy, E, on the conversion degree,<span style='font-size:10.0pt; font-family:"SymbolProp BT";mso-bidi-font-family:"SymbolProp BT"'>a, should be established. If Edepends on<span style='font-size:10.0pt;font-family:Symbol;mso-bidi-font-family:Symbol'>a, Diefallah's composite integral method leads to erroneous results. If Edoes not depend on<span style='font-size: 10.0pt;font-family:"SymbolProp BT";mso-bidi-font-family:"SymbolProp BT"'>a, the true kinetic model should be comprised in the pre-established set of kinetic models. These observations were checked for two sets of non-isothermal data, namely: (a) the TG curves corresponding to the dehydration of CaC2O4·H2O; (b) the TG curves corresponding to the thermal decomposition of polyvinyl chloride (PVC).
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Budrugeac, P., Segal, E. On the use of Diefallah's composite integral method for the non-isothermal kinetic analysis of heterogeneous solid-gas reactions. J Therm Anal Calorim 82, 677–680 (2005). https://doi.org/10.1007/s10973-005-0949-2
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-0949-2