Abstract
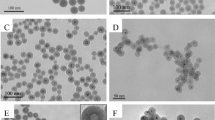
Dumbbell-like SiO2 nanoparticles were synthesized by a simple chemical process in aqueous phase. Prior to the preparation, 3-aminopropyl triethoxysilane (KH550) and 3-chloropropyl triethoxysilane (KH230) were used as modifiers for the surface modification of SiO2 nanoparticles in SiO2 hydrosol. By mixing the SiO2 hydrosol modified by KH550 and KH230, respectively, the dumbbell-like SiO2 nanoparticles were obtained via the reaction between the –NH2 and –CH2Cl groups on the surface of the two SiO2 nanoparticles. The dumbbell-like SiO2 nanoparticles were characterized by dynamic light scattering (DLS), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS). The results showed that the modified SiO2 nanoparticles are covalently coupled in a one-to-one manner. Detailed DLS analysis indicated that about 90% of the single nanoparticles were involved in the coupling reaction and formed new dumbbell-like SiO2 nanoparticles when the ratio of the two kinds of surface modified SiO2 was 1:1. Furthermore, the dumbbell-like SiO2 nanoparticles can be deployed as particle emulsifiers for stabilizing oil-water model systems during emulsification.

Dumbbell-like SiO2 nanoparticles in water phase are successfully fabricated based on nano SiO2 hydrosol by a scalable and controllable chemical process, the new SiO2 nanoparticle hydrosol is expected to be useful as surfactants to reduce the tension between oil and water interface.
Highlights
-
Asymmetric SiO2 nanoparticles with dumbbell-like structure has been successfully synthesized in a one-to-one coupling manner in aqueous phase.
-
About 90% of the single SiO2 nanoparticles were transformed into new dumbbell-like SiO2 nanoparticles in the 1:1 hybrid system.
-
The asymmetric dumbbell-like SiO2 nanoparticles can be used as stabilizers in oil-water system.













Similar content being viewed by others
References
Reguera J, Kim H, Stellacci F (2013) Advances in Janus nanoparticles. Chimia 67:811–818
Kaewsaneha C, Tangboriboonrat P, Polpanich D, Eissa M, Elaissari A (2013) Janus colloidal particles: preparation, properties, and biomedical applications. ACS Appl Mater Interfaces 5:1857–1869
Su H, Hurd Price CA, Jing L, Tian Q, Liu J, Qian K (2019) Janus particles: design, preparation, and biomedical applications. Mater Today Bio 4:100033
Percebom AM, Giner-Casares JJ, Claes N, Bals S, Loh W, Liz-Marzán LM (2016) Janus gold nanoparticles obtained via spontaneous binary polymer shell segregation. Chem Commun 52:4278–4281
Iida R, Kawamura H, Niikura K, Kimura T, Sekiguchi S, Joti Y, Bessho Y, Mitomo H, Nishino Y, Ijiro K (2015) Synthesis of Janus-like gold nanoparticles with hydrophilic/hydrophobic faces by surface ligand exchange and their self-assemblies in water. Langmuir 31:4054–4062
Wu Z, Li L, Liao T, Chen X, Jiang W, Luo W, Yang J, Sun Z (2018) Janus nanoarchitectures: From structural design to catalytic applications. Nano Today 22:62–82
Bhaskar S, Pollock K, Yoshida M, Lahann J (2010) Towards designer microparticles: simultaneous control of anisotropy, shape, and size. Small 6:404–411
Tanaka T, Okayama M, Kitayama Y, Kagawa Y, Okubo M (2010) Preparation of “mushroom-like” Janus particles by site-selective surface-initiated atom transfer radical polymerization in aqueous dispersed systems. Langmuir 26:7843–7847
Mock EB, Zukoski CF (2010) Emulsion polymerization routes to chemically anisotropic particles. Langmuir 26:13747–13750
Deng R, Liu S, Liang F, Wang K, Zhu J, Yang Z (2014) Polymeric Janus particles with hierarchical structures. Macromolecules 47:3701–3707
Zhou P, Wang Q, Zhang C, Liang F, Qu X, Li J, Yang Z (2015) PH responsive Janus polymeric nanosheets. Chin Chem Lett 26:657–661
Baraban L, Makarov D, Streubel R, Monch I, Grimm D, Sanchez S, Schmidt OG (2012) Catalytic Janus motors on microfluidic chip: deterministic motion for targeted cargo delivery. ACS Nano 6:3383–3389
Yuet KP, Hwang DK, Haghgooie R, Doyle PS (2010) Multifunctional superparamagnetic Janus particles. Langmuir 26:4281–4287
Hu J, Zhou S, Sun Y, Fang X, Wu L (2012) Fabrication, properties and applications of Janus particles. Chem Soc Rev 41:4356–4378
Lattuada M, Hatton TA (2011) Synthesis, properties and applications of Janus nanoparticles. Nano Today 6:286–308
Nagao D, Goto K, Ishii H, Konno M (2011) Preparation of asymmetrically nanoparticle-supported, monodisperse composite dumbbells by protruding a smooth polymer bulge from rugged spheres. Langmuir 27:13302–13307
Nagao D, Van kats CM, Hayasaka K, Sugimoto M, Konno M, Imhof A, Blaaderen AV (2010) Synthesis of hollow asymmetrical silica dumbbells with a movable inner core. Langmuir 26:5208–5212
Yang T, Wei L, Jiang L, Liang J, Zhang X, Tang M, Monteiro MJ, Chen Y, Wang Y, Gu S, Zhao D, Yang H, Liu J, Max Lu GQ (2017) Dumbbell-shaped bi-component Mesoporous Janus solid nanoparticles for biphasic interface catalysis. Angew Chem Int Ed 56::8459–8463
Liu S, Guo S, Sun S, You X (2015) Dumbbell-like Au-Fe3O4 nanoparticles: a new nanostructure for supercapacitors. Nanoscale 7:4890–4893
Wei Q, Xiang Z, He J, Wang G, He L, Qian Z, Yang M (2010) Dumbbell-like Au-Fe3O4 nanoparticles as label for the preparation of electrochemical immunosensors. Biosens Bioelectron 26:627–631
Reculusa S, Poncet-Legrand C, Perro A, Duguet E, Bourgeat-Lami E, Mingotaud C, Ravaine S (2005) Hybrid dissymmetrical colloidal particles. Chem Mater 17:3338–3344
Yu H, Chen M, Rice PM, Wang SX, White RL, Sun S (2005) Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett 5:379–382
Kanai T, Nakai H, Yamada A, Fukuyama M, Weitz DA (2019) Preparation of monodisperse hybrid gel particles with various morphologiesvia flow rate and temperature control. Soft Matter 15:6934–6937
Luo J, Yang J, Li Y, He L, Jiang B (2018) Synthesis of amphiphilic silica nanoparticles with double-sphere morphology. Chem J Chin U 39:2170–2177
He M, Wang P, Xiao P, Jia X, Luo J, Jiang B, Xiao B (2022) Synthesis of amphiphilic dumbbell-like janus nanoparticles through one-step coupling. Nanocomposites 8:175–183
Maity N, Basu S, Mapa M, Rajamohanan P, Ganapathy S, Gopinath C, Bhaduri S, Lahiri G (2006) Effect of spacer groups on the performance of MCM-41-supported platinum cluster-derived hydrogenation catalysts. J Catal 242:332–339
Zhang X, Zhao N, Wei W, Sun Y (2006) Chemical fixation of carbon dioxide to propylene carbonate over amine-functionalized silica catalysts. Catal Today 115:102–1062
Adam F, Osman H, Hello KM (2009) The immobilization of 3-(chloropropyl)triethoxysilane onto silica by a simple one-pot synthesis. J Colloid Interface Sci 331:143–147
Ek S, Root A, Peussa M, Niinisto L (2001) Determination of the hydroxyl group content in silica by thermogravimetry and a comparison with 1H MAS NMR results. Thermochim Acta 379:201–212
Aydin F, Uppaladadium G, Dutt M (2016) Harnessing steric hindrance to control interfacial adsorption of patchy nanoparticles onto hairy vesicles. Colloids Surf B 141:458–466
Kim JW, Kim JH, Deaton R (2011) DNA-linked nanoparticle building blocks for programmable matter. Angew Chem Int Ed 50:9185–9190
Mori T, Okada Y, Kamiya H (2016) Effect of surface modification of silica particles on interaction forces and dispersibility in suspension. Adv Powder Technol 27:830–838
Parvole J, Chaduc I, Ako K, Spalla O, Thill A, Ravaine S, Bourgeat-Lami E (2012) Efficient synthesis of snowman- and dumbbell-like silica/polymer anisotropic heterodimers through emulsion polymerization using a surface-anchored cationic initiator. Macromolecules 45:7009–7018
Briard P, Liu Z, Cai X (2020) Measurement of the mean aspect ratio and two characteristic dimensions of polydisperse arbitrary shaped nanoparticles, using translational-rotational ultrafast image-based dynamic light scattering. Nanotechnology 31:395709–395717
Ortega A, Garcı́a de la Torre J (2003) Hydrodynamic properties of rod-like and disk-like particles in dilute solution. J Chem Phys 119:9914–9919
Alexander M, Dalgleish DG (2006) Dynamic light scattering techniques and their applications in food science. Food Biophysics 1:2–13
Barnett CE (1942) Some applications of wave-length turbidimetry in the infrared. J Phys Chem 46:69–75
Weatherston JD, Worstell NC, Wu HJ (2016) Quantitative surface-enhanced Raman spectroscopy for kinetic analysis of aldol condensation using Ag–Au core-shell nanocubes. Analyst 141:6051–6060
Manyà JJ, Azuara M, Manso JA (2018) Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass-Bioenergy 117:115–123
Mydlová J, Krupčík J, Korytár P, Sandra P (2007) On the use of computer assisted resolution of non-separable peaks in a congener specific polybrominated diphenyl ether capillary gas chromatographic analysis. J Chromatogr A 1147:95–104
Acknowledgements
The authors gratefully acknowledge the support from Key Laboratory of Nano Chemistry (KLNC), Petro China. We are thankful to Experimental Testing Center College of Chemistry, Sichuan University for their help in sample analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, X., Xiao, P., Luo, J. et al. Synthesis of asymmetric dumbbell-like SiO2 nanoparticles in aqueous phase and their emulsification properties. J Sol-Gel Sci Technol 105, 152–162 (2023). https://doi.org/10.1007/s10971-022-05984-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05984-w



