Abstract
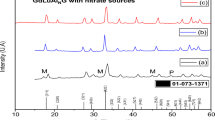
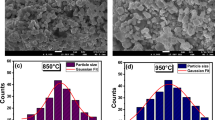
The series M0.5(1+x)FexZr2−x(PO4)3 (M—Ni, Cu, Mn) with Sc2(WO4)3-related structure (SW) were prepared using Pechini technique and characterized by powder X-ray diffraction analysis, SEM, EDX, IR, and Mössbauer spectroscopy. The concentration and temperature limits of solid solution formation were determined, lattice parameters were calculated, and their dependencies on the phosphate’s composition were studied. The crystal structure of the phosphate Mn0.65Fe0.3Zr1.7(PO4)3 (x = 0.3) was refined by the Rietveld method basing on the powder X-ray diffraction data. The compound crystallizes in monoclinic symmetry (space group P21/n) with lattice parameters: a = 8.7941(12), b = 8.9379(16), c = 12.4353(21) Å, β = 90.126(24)°, V = 977.49(27) Å3. Thermal expansion coefficients of the ceramics Cu0.5(1+x)FexZr2−x(PO4)3 were determined. The volume expansion coefficients of the compounds vary in the range (4.4–10.8)∙10−5 K −1.

Highlights
-
New zirconium phosphate ceramics M0.5(1+x)FexZr2-x(PO4)3(M – Ni, Cu, Mn; structural type SW) were synthesized, the formation regions of the solid solutions were revealed.
-
Temperature stability of the phosphates was determined.
-
Crystal structure of the triple phosphate Mn0.65Fe0.3Zr1.7(PO4)3 was refined by Rietveld method.
-
Thermal expansion behavior was studied by XRD, possible reasons were discussed.









Similar content being viewed by others
References
Wang Y, Zhou Y, Song Y, Yang L, Liu F (2018) Mechanical and thermal expansion studies on Ca0.5Sr0.5Zr4−xTixP6O24 ceramics. Ceram Int 44:16698–16702. https://doi.org/10.1016/j.ceramint.2018.06.097
Pet’kov VI, Shipilov AS, Dmitrienko AS, Alekseev AA (2018) Characterization and controlling thermal expansion of materials with kosnarite and langbeinite-type structures. J Ind Eng Chem 57:236–243. https://doi.org/10.1016/j.jiec.2017.08.029
Shi XW, Lian H, Yan XS, Qi R, Yao N, Li T (2016) Fabrication and properties of polyimide composites filled with zirconium tungsten phosphate of negative thermal expansion. Mater Chem Phys 179:72–79. https://doi.org/10.1016/j.matchemphys.2016.05.011
Watanabe H, Tani J, Kido H, Mizuuchi K (2008) Thermal expansion and mechanical properties of pure magnesium containing zirconium tungsten phosphate particles with negative thermal expansion. Mater Sci Eng: A 494:291–298. https://doi.org/10.1016/j.msea.2008.04.037
Tantri P, Ushadevi S, Ramasesha SK (2002) High temperature X-ray studies on barium and strontium zirconium phosphate based low thermal expansion materials. Mater Res Bull 37:1141–1147. https://doi.org/10.1016/S0025-5408(02)00734-1
He S, Xu Y, Zhang B, Sun X, Chen Y, Jin Y (2018) Unique rhombus-like precursor for synthesis of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte with high ionic conductivity. Chem Eng J 345:83–491. https://doi.org/10.1016/j.cej.2018.03.151
Qiu S, Wu X, Wang M, Lucero M, Wang Y, Wang J, Yang Z, Xu W, Wang Q, Gu M, Wen J, Huang Y, Xu ZJ, Feng Z (2019) NASICON–type Na3Fe2(PO4)3 as a low-cost and high-rate anode material for aqueous sodium-ion batteries. Nano Energy 64:103941. https://doi.org/10.1016/j.nanoen.2019.103941
Liu Q, Yan H, Su YU, Shi S, Ye J (2014) Energy transfer studies of dye chromophores in modified zirconium phosphate framework. J Lumin 152:238–240. https://doi.org/10.1016/j.jlumin.2014.01.077
Kanunov AE, Gorshkova EN, Orlova AI, Shushunov AN, Mikheeva ER, Pleskova SN (2013) Elaboration of luminescent materials on the basis of phosphates of NaZr2(PO4)3-type for research of living systems. Phys Procedia 44:224–230. https://doi.org/10.1016/j.phpro.2013.04.027
Zhang Z, Liu L, Zhang X, Zhang J, Zhang W, Wang D (2015) Preparation and investigation of CaZr4(PO4)6:Dy3+ single-phase full-color phosphor. Spectrochim Acta A 137:1–6. https://doi.org/10.1016/j.saa.2014.07.052
Ermilova MM, Sukhanov MV, Borisov RS, Orekhova NV, Pet’kov VI, Novikova SA, Il’in AB, Yaroslavtsev AB (2012) Synthesis of the new framework phosphates and their catalytic activity in ethanol conversion into hydrocarbons. Catal Today 193:37–41. https://doi.org/10.1016/j.cattod.2012.02.029
Shchelokov I, Asabina E, Sukhanov M, Ermilova M, Orekhova N, Pet’kov V, Tereshchenko G (2008) Synthesis, surface properties and catalytic activity of phosphates Cu0.5(1+y)FeyZr2–y(PO4)3 in methanol conversion. Solid State Sci 10:513–517. https://doi.org/10.1016/j.solidstatesciences.2007.12.005
Zhou M, Jiang X, Xia M, Huang H, Lin Z, Yao J, Wu Y(2016) A new congruent-melting double phosphate PbCd(PO3)4 with photocatalytic activity J Alloy Compd 689:599–605. https://doi.org/10.1016/j.jallcom.2016.08.011
Pavlova SN, Sadykov VA, Zabolotnaya GV, Kochubey DI, Maximovskaya RI, Zaikovskii VI, Kriventsov VV, Tsybulya SV, Burgina EB, Volodin AM, Chaikina MV, Kuznetsova NN, Lunin VV, Agrawal D, Roy R (2000) The novel acid catalysts-framework zirconium phosphates: the bulk and surface structure. J Mol Catal A: Chem 158:319–323. https://doi.org/10.1016/S1381-1169(00)00098-4
Pet’kov V, Asabina E, Loshkarev V, Sukhanov M (2016) Systematic investigation of the strontium zirconium phosphate ceramic form for nuclear waste immobilization. J Nucl Mater 471:122–128. https://doi.org/10.1016/j.jnucmat.2016.01.016
Chakraborty N, Basu D, Fischer W (2005) Thermal expansion of Ca1–xSrxZr4(PO4)6 ceramics. J Eur Ceram Soc 25:1885–1893. https://doi.org/10.1016/j.jeurceramsoc.2004.06.019
Asabina E, Pet’kov V, Mayorov P, Lavrenov D, Schelokov I, Kovalsky A (2017) Synthesis, structure and thermal expansion of the phosphates M0.5+xM′xZr2–x(PO4)3 (M, M′–metals in oxidation state +2). Pure Appl Chem 89:523–534. https://doi.org/10.1515/pac-2016-1005
Toh WD, Xu B, Jia J, Chin CS, Chiew J, Gao Z (2017) Lithium iron phosphate (LiFePO4) battery power system for deepwater emergency operation. Energy Procedia 143:348–353. https://doi.org/10.1016/j.egypro.2017.12.695
La Parola V, Liveri VT, Todaro L, Lombardo D, Bauer EM, Dell’Era A, Longo A, Caschera D, de Caro T, Grazia Toro R, Calandra P (2018) Iron and lithium–iron alkyl phosphates as nanostructured material for rechargeable batteries. Mater Lett 220:58–61. https://doi.org/10.1016/j.matlet.2018.02.112
Marques CF, Olhero S, Abrantes JCC, Marote A, Ferreira S, Vieira SI, Ferreira JMF (2017) Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram Int 43:15719–15728. https://doi.org/10.1016/j.ceramint.2017.08.133
Marques CF, Perera FH, Marote A, Ferreira S, Vieira SI, Olhero S, Miranda P, Ferreira JMF (2017) Biphasic calcium phosphate scaffolds fabricated by direct write assembly: mechanical, anti-microbial and osteoblastic properties. J Eur Ceram Soc 37:359–368. https://doi.org/10.1016/j.jeurceramsoc.2016.08.018
Gadamsetti S, Mathangi N, Hussain S, Kumar Velisoju V, Chary KVR (2018) Vapor phase esterification of levulinic acid catalyzed by γ-Al2O3 supported molybdenum phosphate catalysts. Mol Catal 451:192–199. https://doi.org/10.1016/j.mcat.2018.01.011
Song X, Sun Q, Gao L, Chen W, Wu Y, Li Y, Mao L, Yang J–H (2018) Nickel phosphate as advanced promising electrochemical catalyst for the electro-oxidation of methanol. Int J Hydrog Energy 43:12091–12102. https://doi.org/10.1016/j.ijhydene.2018.04.165
Pet’kov VI, Sukhanov MV, Ermilova MM, Orekhova NV, Tereshchenko GF (2010) Development and synthesis of bulk and membrane catalysts based on framework phosphates and molybdates. Russ J Appl Chem 83:1731–1741. https://doi.org/10.1134/S1070427210100022
Shahbazi–Alavi H, Nazemzadeh SH, Ziarati A, Safaei–Ghomi J (2018) Nano-NiZr4(PO4)6 as a superior catalyst for the synthesis of propargylamines under ultrasound irradiation. Z Naturforsch B: Chem Sci 73:185–189. https://doi.org/10.1515/znb-2017-0178
Pet’kov VI, Kurazhkovskaya VS, Orlova AI, Spiridonova ML (2002) Synthesis and crystal chemical characteristics of the structure of M0.5Zr2(PO4)3 phosphates. Crystallogr Rep 47:736–743. https://doi.org/10.1134/1.1509386
Asabina EA, Glukhova IO, Pet’kov VI, Borovikova EYU, Koval’skii AM (2017) Synthesis and structure of phosphates M0.5Ti2(PO4)3. Rus J Gen Chem 87:684–689. https://doi.org/10.1134/S1070363217040041
Hagman LO, Kierkegaard P (1968) The crystal structure of NaM2IV(PO4)3; MeIV = Ge, Ti, Zr. Acta Chem Scand 22:1822–1832. https://doi.org/10.3891/acta.chem.scand.22-1822
Oikonomou P, Dedeloudis CH, Stournaras CJ, Ftikos CH (2007) [NZP]: a new family of ceramics with low thermal expansion and tunable properties. J Eur Ceram Soc 27:1253–1258. https://doi.org/10.1016/j.jeurceramsoc.2006.04.021
Bortsova YEV, Koryttseva AK, Orlova AI, Kurazhkovskaya VS, Kаzantsev GN, Samoilov SG, Kаruchkina YEA (2009) New NZP–phosphates B0.5FeTa(PO4)3 (where B—Ca, Sr, Ba): Synthesis, crystallochemical investigation and thermal expansion. J Alloy Compds 475:74–78. https://doi.org/10.1016/j.jallcom.2008.07.062
Oota T, Yamai I (1986) Thermal expansion behavior of NaZr2(PO4)3 type compounds. J Am Ceram Soc 69:1–6. https://doi.org/10.1111/j.1151-2916.1986.tb04682.x
Zhu Y, Kanamori K, Moitra N, Kadono K, Ohi S, Shimobayashi N, Nakanishi K (2016) Metal zirconium phosphate macroporous monoliths: versatile synthesis, thermal expansion and mechanical properties. Microporous Mesoporous Mater 225:122–127. https://doi.org/10.1016/j.micromeso.2015.12.002
Tereshchenko GF, Orekhova NV, Ermilova MM, Malygin AA, Orlova AI (2006) Nanostructured phosphorus–oxide-containing composite membrane catalysts. Catal Today 118:85–89. https://doi.org/10.1016/j.cattod.2005.12.014
Pylinina AI, Chernyshova MN, Lobanov NN, Kasatkin EM, Savilov SV (2017) Dehydration of isobutyl alcohol on cesium-cobalt-containing NASICON catalysts. Theor Exp Chem 53:47–52. https://doi.org/10.1007/s11237-017-9500-3
Pylinina AI, Mikhalenko II (2011) Dehydrogenation of butyl alcohols on NASICON-type solid electrolytes of Na1–2xCuxZr2(PO4)3 composition. Russ J Phys Chem 85:2109–2114. https://doi.org/10.1134/S0036024411110252
Serghini A, Brochu R, Ziyad M, Loukah M, Védrine JC (1991) Behaviour of copper–zirconium nasicon-type phosphate, CuIZr2(PO4)3, in the decomposition of isopropyl alcohol. J Chem Soc, Faraday Trans 87:2487–2491. https://doi.org/10.1039/FT9918702487
Serghini A, Brochu R, Ziyad M, Védrine JC (1992) Synthesis, characterization and catalytic behaviour of Cu0.5M2(PO4)3 (M = Zr, Sn, Ti). J Alloy Compd 188:60–64. https://doi.org/10.1016/0925-8388(92)90643-N
Glukhova IO, Asabina EA, Pet’kov VI, Mironova EYU, Zhilyaeva NA, Kovalyskii AM, Yaroslavtsev AB (2020) Zirconium d-transition metal phosphates as catalysts for selective dehydration of methanol to dimethyl ether. Inorg Mater 56:395–401. https://doi.org/10.1134/S0020168520040056
Matraszek A, Godlewska P, Macalik L, Hermanowicz K, Hanuza J, Szczygie I (2015) Optical and thermal characterization of microcrystalline Na3RE(PO4)2:Yb orthophosphates synthesized by Pechini method (RE = Y, La, Gd). J Alloy Compd 619:275–283. https://doi.org/10.1016/j.jallcom.2014.08.189
Mohassel R, Sobhani A, Salavati–Niasari M, Goudarzi M (2018) Pechini synthesis and characteristics of Gd2CoMnO6 nanostructures and its structural, optical and photocatalytic properties. Spectrochim Acta A 204:232–240. https://doi.org/10.1016/j.saa.2018.06.050
Verma S, Rani S, Kumar S, Majeed Khan MA (2018) Rietveld refinement, micro-structural, optical and thermal parameters of zirconium titanate composites. Ceram Int 44:1653–1661. https://doi.org/10.1016/j.ceramint.2017.10.090
Fahami A, Nasiri–Tabrizi B, Beall GW, Basirun WJ (2017) Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement. Chin J Chem Eng 25:238–247. https://doi.org/10.1016/j.cjche.2016.07.013
Kim Y–Il, Izumi F (1994) Structure refinements with a new version of the Rietveld–refinement program RIETAN. J Ceram Soc Jpn 102:401–404. https://doi.org/10.2109/jcersj.102.401
Jouanneaux A, Verbaere A, Piffard Y (1991) How to distinguish between monoclinic distortions of NASICON and Sc2(WO4)3 structure types from X-ray-powder patterns-crystal-structure of Ni0.5Zr2(PO4)3. Eur J Solid State Inorg Chem 28:683–699
Pet’kov V, Asabina E, Markin A, Smirnova NN, Kitaev DB (2005) Thermodynamic data of the NZP compounds family. J Therm Anal Calorim 80:695–700. https://doi.org/10.1007/s10973-005-0716-4
Asabina E, Glukhova I, Butrina O, Pet’kov V, Kovalsky A (2018) Synthesis and crystal structure of phosphates Zn0.5(1+x)FexM2-x(PO4)3 (M = Zr, Hf). J Alloy Compd 741:1229–1234. https://doi.org/10.1016/j.jallcom.2018.01.226
Menil F (1985) Systematic trends of the Fe57 Mössbauer isomer shifts in (FeOn) and (FeFn) polyhedra. Evidence of a new correlation between the isomer shift and the inductive effect of the competing bond T–X ( → Fe) (where X is O or F and T any element with a formal positive charge). J Phys Chem Solids 7:763–789. https://doi.org/10.1016/0022-3697(85)90001-0
Orlova A (2002) Isomorphism in crystalline phosphates of the NaZr2(PO4)3 structural type and radiochemical problems. Radiochem 44:423–445. https://doi.org/10.1023/A:1021192605465
Pet’kov VI, Orlova AI (2003) Crystal-chemical approach to predicting the thermal expansion of compounds in the NZP family. Inorg Mater 39:1013–1023. https://doi.org/10.1023/A:1026074722220
Gobechiya ER, Sukhanov MV, Pet’kov VI, Kabalov YUK (2008) Crystal structure of the double magnesium zirconium orthophosphate at temperatures of 298 and 1023 K. Cryst Rep 53:53–59. https://doi.org/10.1134/S1063774508010069
Xie DY, Wang ZH, Liu XS, Song WB, Yuan BH, Liang EJ (2012) Rapid synthesis of low thermal expansion materials of Ca1–xSrxZr4P6O24. Ceram Int 38:3807–3813. https://doi.org/10.1016/j.ceramint.2012.01.029
Dean–Mo L, Brown JJ (1993) Thermal expansion of porous (Ca1–xMgx)Zr4(PO4)6 ceramics. Mater Chem Phys 33:43–49. https://doi.org/10.1016/0254-0584(93)90088-4
Wang Y, Zhou Y, Song Y, Yang L, Liu F (2018) Mechanical and thermal expansion studies on Ca0.5Sr0.5Zr4-xTixP6O24 ceramics. Ceram Int 44:16698–16702. https://doi.org/10.1016/j.ceramint.2018.06.097
Pet’kov VI, Orlova AI, Kasantsev GN, Samoilov SG, Spiridonova ML (2001) thermal expansion in the Zr and 1-, 2-valent complex phosphates of NaZr2(PO4)3 (NZP) Structure. J Therm Anal Calorim 66:623–632. https://doi.org/10.1023/A:1013145807987
Govindan Kutty KV, Asuvathraman R, Sridharan R (1998) Thermal expansion studies on the sodium zirconium phosphate family of compounds A1/2M2(PO4)3: effect of interstitial and framework cations. J Mater Sci 33:4007–4013. https://doi.org/10.1023/A:1004661132398
Limaye SY, Agrawal DK, McKinstry HA (1987) Synthesis and thermal expansion of MZr4P6O24 (M = Mg, Ca, Sr, Ba). J Am Ceram Soc 70:232–236. https://doi.org/10.1111/j.1151-2916.1987.tb04884.x
Acknowledgements
The reported study was funded by Russian Fund of Basic Research (RFBR) according to the research project No. 18-29-12063 “Advanced mineral-like ceramics with improved service characteristics for an effective immobilization of toxic and radioactive elements” and by the Russian Science Foundation (Agreement number 20-79-10286) in the part of the characterization of materials (Andrey Kovalskii).
Author contribution
IG prepared and characterized the samples by XRD and IR methods, wrote the original draft of the manuscript. EA designed and supervised this work, co-wrote the manuscript. VP co-wrote, reviewed, and edited the manuscript. AK carried out SEM and EDX studies. KP performed Mössbauer spectroscopy experiments and processed the obtained data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Glukhova, I., Asabina, E., Pet’kov, V. et al. Synthesis, structural characteristics, and thermal expansion behavior of zirconium phosphate ceramics with d-transition metals. J Sol-Gel Sci Technol 99, 354–365 (2021). https://doi.org/10.1007/s10971-021-05577-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05577-z