Abstract
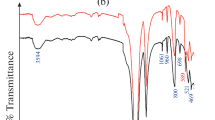
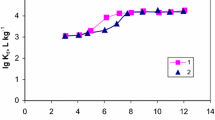
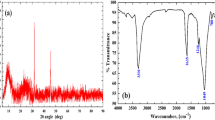
Iron phosphate@talc (IPT) sorbent was fabricated by precipitation method and utilized for 137Cs, 152+154Eu, and 131Ba sorption from aqueous media. Different analytical apparatuses such as XRD, FT-IR, SEM, and XRF were used to find the structure, morphology, and functional groups of IPT sorbent. Different parameters like shaking time, pH, temperature, and initial metal concentrations were studied. The obtained data reveal that the sorption of 137Cs, 152+154Eu, and 131Ba was pH, time-dependence, and kinetics following pseudo-2nd-order kinetics. The Langmuir and Freundlich equations were used as model isotherms. Thermodynamics parameters were calculated to prove that the sorption reaction was spontaneous and endothermic.









Similar content being viewed by others
Availability of data and materials
Yes.
References
Zhang H, Zhu M, Du X et al (2021) Removal of cesium from radioactive waste liquids using geomaterials. Appl Sci 11:8407
Mezga LJ (1990) Standardization of radioactive waste categories. Oak Ridge Gaseous Diffusion Plant, TN (USA)
Hamed MM, Holiel M, Ismail ZH (2016) Removal of 134Cs and 152+154Eu from liquid radioactive waste using Dowex HCR-S/S. Radiochim Acta 104:399–413
Roy K, Pal DK, Basu S, Nayak D, Lahiri S (2002) Synthesis of a new ion exchanger, zirconiums vanadate, and its application to the separation of barium and cesium radionuclides at tracer levels. Appl Radiat Isot Isot 57:471–475
Hassan HS, Kenawy SH, El-Bassyouni GT et al (2020) Sorption behavior of cesium and europium radionuclides onto nano-sized calcium silicate. Part Sci Technol 38:105–112
Majidnia Z, Idris A, Majid M et al (2015) Efficiency of barium removal from radioactive wastewater using the combination of maghemite and titania nanoparticles in PVA and alginate beads. Appl Radiat Isot 105:105–113
Abass MR, El-Masry EH, El-Kenany WM (2022) Gamma irradiation-induced preparation of polyacrylonitrile acrylamide nano-silica for removal of some hazardous metals. J Inorg Organomet Polym Mater 32:536–546
Eid MA, Abass MR, El-Kenany WM (2022) Fabrication and application of nanosized stannic oxide for sorption of some hazardous metal ions from aqueous solutions. Radiochim Acta 110:1003–1015
Hodkin DJ, Stewart DI, Graham JT, Burke IT (2016) Coprecipitation of 14C and Sr with carbonate precipitates: the importance of reaction kinetics and recrystallization pathways. Sci Total Environ 562:335–343
Jia F, Yin Y, Wang J (2018) Removal of cobalt ions from simulated radioactive wastewater by vacuum membrane distillation. Prog Nucl Energy 103:20–27
Chiera NM, Bolisetty S, Eichler R et al (2021) Removal of radioactive cesium from contaminated water by whey protein amyloids-carbon hybrid filters. RSC Adv 11:32454–32458
Hurtado-Bermúdez S, Villa-Alfageme M, Mas JL, Alba MD (2018) Comparison of solvent extraction and extraction chromatography resin techniques for uranium isotopic characterization in high-level radioactive waste and barrier materials. Appl Radiat Isot 137:177–183
Naeimi S, Faghihian H (2017) Performance of novel adsorbent prepared by magnetic metal-organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Sep Purif Technol 175:255–265
Zhang L, Wei J, Zhao X et al (2015) Adsorption characteristics of strontium on synthesized antimony silicate. Chem Eng J 277:378–387
Hassan SSM, Kamel AH, Youssef MA et al (2020) Removal of barium and strontium from wastewater and radioactive wastes using a green bioadsorbent, Salvadora persica (Miswak). Desalin Water Treat 192:306–314
Xie H, Zhang S, Zhong L et al (2021) Effect of the occurrence state of magnesium in talc on the adsorption of Pb(II). J Alloys Compd 887:161288
Abass MR, El-Kenany WM, El-Masry EH (2022) High efficient removal of lead(II) and cadmium(II) ions from multi-component aqueous solutions using polyacrylic acid acrylonitrile talc nanocomposite. Environ Sci Pollut Res 29:72929–72945
Sani HA, Ahmad MB, Saleh TA (2016) Synthesis of zinc oxide/talc nanocomposite for enhanced lead adsorption from aqueous solutions. RSC Adv 6:108819–108827
Hagag MS, Esmaeel SM, Salem F et al (2022) Uranium sorption from waste solutions by Talc Phosphogypsum ferri-silicate synthetic new sorbent. Radiochim Acta 110:93–106
Basuki T, Nakashima S (2021) Cs adsorption and CsCl particle formation facilitated by amino talc-like clay in aqueous solutions at room temperature. ACS Omega 6:26026–26034
Sprynskyy M, Kowalkowski T, Tutu H et al (2011) Adsorption performance of talc for uranium removal from aqueous solution. Chem Eng J 171:1185–1193
Kalantari K, Ahmad MB, Masoumi HRF et al (2014) Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology. Int J Mol Sci 15:12913–12927
Wenlei L, Shanlin Z, Shuang C et al (2014) Adsorptive characteristics of modified talcum powder in removing methylene blue from wastewater. Chem Speciat Bioavailab 26:167–175
Abass MR, Maree RM, Sami NM (2022) Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. Int J Environ Anal Chem 1–20
Abass MR, Ibrahim AB, Abou-Mesalam MM (2021) Retention and selectivity behavior of some lanthanides using bentonite dolomite as a natural material. Chem Pap 75:3751–3759
Hamed MM, Shahr El-Din AM, Abdel-Galil EA (2019) Nanocomposite of polyaniline functionalized Tafla: synthesis, characterization, and application as a novel sorbent for selective removal of Fe(III). J Radioanal Nucl Chem 322:663–676
Abass MR, Ibrahim AB, El-Masry EH, Abou-Mesalam MM (2021) Optical properties enhancement for polyacrylonitrile-ball clay nanocomposite by heavy metals saturation technique. J Radioanal Nucl Chem 329:849–855
Kasem AE, Abdel-Galil EA, Belacy N, Badawy NA (2021) Kinetics and adsorption equilibrium of some radionuclides on polyaniline/SiO2 composite. Radiochim Acta 109:85–97
Gupta VK, Agarwal S, Tyagi I et al (2015) Synthesis, characterization and analytical application of cellulose acetate tin(IV) molybdate nanocomposite ion exchanger: binary separation of heavy metal ions and antimicrobial activity. Ionics 21:2069–2078
Hassan RS, Abass MR, Eid MA, Abdel-Galil EA (2021) Sorption of some radionuclides from liquid waste solutions using anionic clay hydrotalcite sorbent. Appl Radiat Isot 178:109985
Metwally SS, Hassan HS, Samy NM (2019) Impact of environmental conditions on the sorption behavior of 60Co and 152+154Eu radionuclides onto polyaniline/zirconium aluminate composite. J Mol Liq 287:110941
Mahrous SS, Abdel-Galil EA, Mansy MS (2022) Investigation of modified orange peel in the removal of Cd2+, Co2+ and Zn2+ from wastewater. J Radioanal Nucl Chem 331:985–997
Abou-Mesalam MM, Abass MR, Abdel-Wahab MA et al (2018) Polymeric composite materials based on silicate: II. sorption and distribution studies of some hazardous metals on irradiated doped polyacrylamide acrylic acid. Desalin Water Treat 109:176–187
Hassan RS, Eid MA, El-Sadek AA, Mansy MS (2019) Preparation, characterization and application of Mg/Fe Hydrotalcite as gamma sealed source for spectroscopic measurements. Appl Radiat Isot 151:74–80
Mansy MS, Hassan RS, Selim YT, Kenawy SH (2017) Evaluation of synthetic aluminum silicate modified by magnesia for the removal of 137Cs, 60Co and 152+154Eu from low-level radioactive waste. Appl Radiat Isot 130:198–205
Comodi P, Zanazzi PF (1993) Structural study of ellenbergerite. Part II: effects of high pressure. Eur J Miner 5:831–838
Taha KK, Suleiman TM, Musa MA (2011) Performance of Sudanese activated bentonite in bleaching cottonseed oil. J Bangladesh Chem Soc 24:191–201
Andrunik M, Bajda T (2019) Modification of bentonite with cationic and nonionic surfactants: structural and textural features. Materials 12:3772
Alabarse FG, Conceição RV, Balzaretti NM et al (2011) In-situ FTIR analyses of bentonite under high-pressure. Appl Clay Sci 51:202–208
Rao KTV, Souzanchi S, Yuan Z et al (2017) Simple and green route for preparation of tin phosphate catalysts by solid-state grinding for dehydration of glucose to 5-hydroxymethylfurfural (HMF). RSC Adv 7:48501–48511
Xu Y, Zhou F, Chen M et al (2020) Facile assembly of 2D α-zirconium phosphate supported silver nanoparticles: superior and recyclable catalysis. New J Chem 44:9793–9801
Abdel-Galil EA, Eid MA, Hassan RS (2020) Preparation of nanosized stannic silicomolybdate for chromatographic separation of Y(III) from Zr(IV). Part Sci Technol 38:113–120
Abdel-Galil EA, Ibrahim AB, El-Kenany WM (2021) Facile fabrication of a novel silico vanadate ion exchanger: evaluation of its sorption behavior towards europium and terbium ions. Desalin Water Treat 226:303–318
Ibrahim AB, Abass MR, EL-Masry EH, Abou-Mesalam MM, (2021) Gamma radiation-induced polymerization of polyacrylic acid-dolomite composite and applications for removal of cesium, cobalt, and zirconium from aqueous solutions. Appl Radiat Isot 178:109956
Abou-Mesalam MM, Abass MR, Abdel-Wahab MA et al (2016) Complex doping of d-block elements cobalt, nickel, and cadmium in magneso-silicate composite and its use in the treatment of aqueous waste. Desalin Water Treat 57:25757–25764
Metwally SS, Hassan RS, El-Masry EH, Borai EH (2018) Gamma-induced radiation polymerization of kaolin composite for sorption of lanthanum, europium and uranium ions from lowgrade monazite leachate. J Radioanal Nucl Chem 315:39–49
Moloukhia H (2010) Use of animal charcoal prepared from the bivalve chaelatura (chaelatura) companyoi in treatment of waste solution containing cesium and strontium ions. J Radiat Res Appl Sci 3:343–356
Abou-Mesalam MM, Abass MR, Ibrahim AB, Zakaria ES (2020) Polymeric composite materials based on silicate. III-Capacity and sorption behavior of some hazardous metals on irradiated doped polyacrylamide acrylonitrile. Desalin Water Treat 193:402–413
El-Naggar IM, Sheneshen ES, Abdel-Galil EA (2016) Diffusion mechanism of Co2+, Cu2+, Cd2+, Cs+, and Pb2+ ions in the particles of polyaniline silicotitanate. Part Sci Technol 34:373–379
Sheha RR, El-Zahhar AA (2008) Synthesis of some ferromagnetic composite resins and their metal removal characteristics in aqueous solutions. J Hazard Mater 150:795–803
Borai EH, Attallah MF, Elgazzar AH, El-Tabl AS (2019) Isotherm and kinetic sorption of some lanthanides and iron from aqueous solution by aluminum silicotitante exchanger. Part Sci Technol 37:414–426
Abdel-Galil EA, Ibrahim AB, Abou-Mesalam MM (2016) Sorption behavior of some lanthanides on polyacrylamide stannic molybdophosphate as organic-inorganic composite. Int J Ind Chem 7:231–240
El-Deen SEAS, Moussa SI, Mekawy ZA et al (2017) Evaluation of CNTs/MnO2 composite for adsorption of 60Co(II), 65Zn(II) and Cd(II) ions from aqueous solutions. Radiochim Acta 105:43–55
Ahmed IM, Aglan RF, Hamed MM (2017) Removal of Arsenazo-III and Thorin from radioactive waste solutions by adsorption onto low-cost adsorbent. J Radioanal Nucl Chem 314:2253–2262
Karaca S, Gürses A, Açışlı Ö et al (2013) Modeling of adsorption isotherms and kinetics of Remazol Red RB adsorption from aqueous solution by modified clay. Desalin Water Treat 51:2726–2739
Dakroury GA, El-Shazly EAA, Hassan HS (2021) Preparation and characterization of ZnO/Chitosan nanocomposite for Cs(I) and Sr(II) sorption from aqueous solutions. J Radioanal Nucl Chem 330:159–174
Manjuladevi M, Anitha R, Manonmani S (2018) Kinetic study on adsorption of Cr(VI), Ni(II), Cd(II) and Pb(II) ions from aqueous solutions using activated carbon prepared from Cucumis melo peel. Appl Water Sci 8:1–8
Abass MR, El-Masry EH, Ibrahim AB (2021) Preparation, characterization, and applications of polyacrylonitrile/ball clay nanocomposite synthesized by gamma radiation. Environ Geochem Health 43:3169–3188
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Fard AK, Mckay G, Chamoun R et al (2017) Barium removal from synthetic natural and produced water using MXene as two dimensional (2-D) nanosheet adsorbent. Chem Eng J 317:331–342
Abass MR, Breky MME, Maree RM (2022) Removal of 137Cs and 90Sr from simulated low-level radioactive waste using tin(IV) vanadate sorbent and its potential hazardous parameters. Appl Radiat Isot 189:110417
Mahrous SS, Mansy MS, Abdel Galil EA (2022) Decontamination of 137Cs, 95Zr, 154Eu and 144Ce from aqueous solutions using polyacrylamide titanium tungstosilicate. J Radioanal Nucl Chem 1–14
Omar HA, Moloukhia H (2008) Use of activated carbon in removal of some radioisotopes from their waste solutions. J Hazard Mater 157:242–246
Abass MR, Ibrahim AB, Abou-Mesalam MM (2022) Sorption and selectivity behavior of some rare earth elements on bentonite-dolomite composites as natural materials. Radiochemistry 64:349–359
Abdel-Galil EA, Hassan RS, Eid MA (2019) Assessment of nano-sized stannic silicomolybdate for the removal of 137Cs, 90Sr, and 141Ce radionuclides from radioactive waste solutions. Appl Radiat Isot 148:91–101
Mahrous SS, Abass MR, Mansy MS (2022) Bentonite phosphate modified with nickel: preparation, characterization, and application in the removal of 137Cs and 152+154Eu. Appl Radiat Isot 190:110445
El-Naggar IM, Mowafy EA, Abdel-Galil EA, El-Shahat MF (2010) Synthesis, characterization and ion-exchange properties of a novel ‘organic–inorganic’ hybrid cation-exchanger: polyacrylamide Sn(IV) molybdophosphate. Glob J Phys Chem 1:91–106
Acknowledgements
This work has been supported by the Egyptian Atomic Energy Authority.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Muhammad S. Mansy: experimental work, reviewing, and editing. Marwa A. Eid: experimental work and editing. Mohamed M.E. Breky: experimental work and editing. Mohamed R. Abass: Data curation, preparing sorbent, writing—original draft review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Consent to publish
Yes.
Consent to participate
Yes.
Ethical approval
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mansy, M.S., Eid, M.A., Breky, M.M.E. et al. Sorption behavior of 137Cs, 152+154Eu and 131Ba from aqueous solutions using inorganic sorbent loaded on talc. J Radioanal Nucl Chem 332, 2971–2987 (2023). https://doi.org/10.1007/s10967-023-08977-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08977-3