Abstract
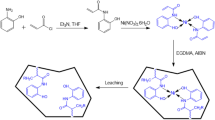
A neodymium-imprinted polymeric sorbent was synthesized to recover the enriched 146Nd-target material from 147Pm production process. The studies indicated the neodymium (III) adsorption capacity of 1.5 mg g− 1. 146Nd-oxide was irradiated in Tehran Research Reactor to produce 147Pm. Then, the prepared Nd-selective sorbent was applied to recover the macro-gram amounts of 146Nd from a micro-gram amount of 147Pm. Regarding the high efficiency of leaching process (> 96%), the remaining natural Nd-ions trapped in polymer structure caused an acceptable slight down-blending of the target material. The sorbent with 146Nd was burned with a heat-gun to produce 146Nd2O3 for the next experiments.








Similar content being viewed by others
References
Flickerm H, Loferski JJ, Elleman TS (1964) Construction of a promethium-147 atomic battery. IEEE Trans Electron Devices 11:2–8
Broderick K, Lusk R, Hinderer J, Griswold J, Boll R, Garland M, Heilbronn L, Mirzadeh S (2018) Reactor production of promethium-147. Appl Radiat Isot 144:54–63
Artun O (2017) Investigation of the production of promethium-147 via particle accelerator. Indian J Phys 91:909–914
Hinderer JH (2010) Radioisotopic impurities in promethium-147 produced at the ORNL high flux isotope reactor. Trans Am Nucl Soc 103:1163–1164
Zell-Lusk RI (2013) Computer code verification and cross section calculation for promethium-147. Nuclear Eng Reports. https://trace.tennessee.edu/utne_reports/1
Sugihara TT, James HI, Troianello EJ, Bowen VT (1959) Radiochemical separation of fission products from large volumes of sea water. Strontium, cesium, cerium, and promethium. Anal Chem 31:44–49
Pressly RS, Ottinger CL, Orr PB, Beauchamp EE (1960) Purification of kilocurie quantities of promethium-147 by ion exchange. Report ORNL-2928
Lee CS, Wang YM, Cheng WL, Ting G (1989) Chemical study on the separation and purification of promethium-147. J Radioanal Nucl Chem 130:21–37
Knapp FE, Boll RA, Mirzadeh S (2008) Reactor production and purification of promethium-147. United State Patent, No: US 7435399 B2
Monroy-Guzman F, Jaime Salinas E (2015) Separation of micro-macrocomponent systems: 149Pm–Nd, 161Tb–Gd, 166Ho–Dy and 177Lu–Yb by extraction chromatography. J Mex Chem Soc 59(2):143–150
Baulin VE, Kalashnikova IP, Kovalenko OV, Baulin DV, Usolkin AN, Tsivadze AY (2016) Acidic phosphoryl podands as components of extraction chromatography material for selective extraction of promethium-147. Prot Met Phys Chem Surf 52:996–1004
Ambe F, Burba P, Lieser KH (1978) Separation of lanthanides by ion-exchange equilibria—a comparison of three cases of selective separations. Z Anal Chem 289:96–101
Imura H, Mito H (1995) Selective extraction of light lanthanides (III) with 18-crown-6 and perfluorooctanoate. J Radioanal Nucl Chem 189:229–235
Dehghani F, Wells T, Cotton NJ, Foster NR (1996) Extraction and separation of lanthanides using dense gas CO2 modified with tributyl phosphate and di(2-ethyl hexyl)phosphoric acid. J Supercrit Fluids 9:263–272
Nesterenko PN, Jonesb P (1997) First isocratic separation of fourteen lanthanides and yttrium by high-performance chelation ion chromatography. Anal Commun 34:7–8
Hirayama N, Takeuchi I, Honjo T (1997) Ion-pair extraction system for the mutual separation of lanthanides using divalent quadridentate Schiff bases. Anal Chem 69:4814–4818
Koma Y, Koyama T, Tanaka Y (1999) Enhancement of the mutual separation of lanthanide elements in the solvent extraction based on the CMPO-TBP mixed solvent by using a DTPA-nitrate solution. J Nucl Sci Tech 36:934–939
Araki K, Yoshida M, Uezu K, Goto M, Furusaki S (2000) Lanthanide-imprinted resins prepared by surface template polymerization. J Chem Eng Jpn 33:665–668
Doleža J, Moreno C, Hrdlicka A, Valiente M (2000) Selective transport of lanthanides through supported liquid membranes containing non-selective extractant, di-(2-ethylhexyl) phosphoric acid, as a carrier. J Membr Sci 168:175–181
Kolarik Z (2008) Complexation and separation of lanthanides (III) and actinides (III) by heterocyclic N-donors in solutions. Chem Rev 108:4208–4252
Nishihama S, Tajiri Y, Yoshizuka K (2006) Separation of lanthanides using micro solvent extraction system. Ars Separatoria Acta 4:18–26
Husain M, Ansari SA, Mohapatra PK, Gupta RK, Parmar VS, Manchanda VK (2008) Extraction chromatography of lanthanides using N,N,N′,N′-tetraoctyl diglycolamide (TODGA) as the stationary phase. Desalination 229:294–301
Shimojo K, Aoyagi N, Saito T, Okamura H, Kubota F, Goto M, Naganawa H (2014) Highly efficient extraction separation of lanthanides using a diglycolamic acid extractant. Anal Sci 30:263–269
Nakasea M, Tanakab M, Takeshit K (2015) Continuous mutual separation of lanthanides by a liquid-liquid countercurrent centrifugal extractor with Taylor vortices. In: The fourth international symposium on innovative nuclear energy systems, INES-4, Energy Procedia, vol 71, pp 106–111
Trikha R, Sharma BK, Sabharwal KN, Prabhu K (2015) Elution profiles of lanthanides with α-hydroxyisobutyric acid by ion exchange chromatography using fine resin. J Sep Sci 38:3810–3814
Biju VM, Gladis JM, Rao TP (2003) Effect of γ-irradiation of ion imprinted polymer (IIP) particles for the preconcentrative separation of dysprosium from other selected lanthanides. Talanta 60:747–754
Buyuktiryaki S, Say R, Ersoz A, Birlik E, Denizli A (2005) Selective preconcentration of thorium in the presence of UO(2)(2+), Ce(3+) and La(3+) using Th(IV)-imprinted polymer. Talanta 67:640–645
Alizadeh T, Amjadi S (2013) Synthesis of nano-sized Eu3+-imprinted polymer and its application for indirect voltammetric determination of europium. Talanta 106:431–439
Laatikainen K, Udomsap D, Siren H, Brisset H, Sainio T, Branger C (2015) Effect of template ion-ligand complex stoichiometry on selectivity of ion-imprinted polymers. Talanta 134:538–545
Moussa M, Pichon V, Mariet C, Vercouter T, Delaunay N (2016) Potential of ion imprinted polymers synthesized by trapping approach for selective solid phase extraction of lanthanides. Talanta 161:459–468
Shirvani-Arani S, Ahmadi SJ, Bahrami-Samani A, Ghannadi-Maragheh M (2008) Synthesis of nano-pore samarium (III)-imprinted polymer for preconcentrative separation of samarium ions from other lanthanide ions via solid phase extraction. Anal Chim Acta 623:82–88
Jiajia G, Jibao C, Qingde (2009) S Ion imprinted polymer particles of neodymium: synthesis, characterization and selective recognition. J Rare Earths 27:22–27
Liu QP, Li HZ, Zhuang HY, Pei MS (2011) Synthesis of a new ion imprinted polymer material for separation and preconcentration of traces of neodymium ions. Adv Mater Res 306–307:705–708
Dolak I, Kecili R, Hür D, Ersöz A, Say R (2015) Ion-imprinted polymers for selective recognition of neodymium (III) in environmental samples. Ind Eng Chem Res 54(19):5328–5335
Zheng X, Zhang F, Liu E, Xu X, Yan Y (2017) Efficient recovery of neodymium in acidic system by free-standing dual-template docking oriented ionic imprinted mesoporous films. ACS Appl Mater Interfaces 9:730–739
Zheng X, Zhang Y, Zhang F, Li Z, Yan Y (2018) Dual-template docking oriented ionic imprinted bilayer mesoporous films with efficient recovery of neodymium and dysprosium. J Hazard Mater 353:496–504
Acknowledgements
This work was supported through the grant provided by Nuclear Science and Technology Research Institute (NSTRI). Mr. Ali Yousefi’s assistances is gratefully acknowledged, as well.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shirvani-Arani, S., Hosseini, S.E., Ghannadi-Maragheh, M. et al. Enriched 146Nd recovery from a 147Pm production process by applying ion imprinting technique. J Radioanal Nucl Chem 327, 761–770 (2021). https://doi.org/10.1007/s10967-020-07560-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07560-4