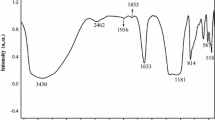
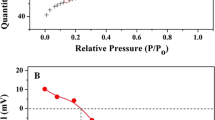
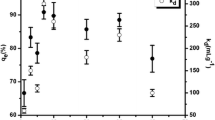
Abstract
In this study, the adsorption of U(VI) from aqueous solution on Na-rectorite was studied as a function of various environmental conditions such as contact time, pH, ionic strength, soil humic acid (HA)/fulvic acid (FA), solid contents, and temperature under ambient conditions by using batch technique. The kinetic adsorption is fitted by the pseudo-second-order model very well. The adsorption of U(VI) on Na-rectorite was strongly dependent on pH and ionic strength. A positive effect of HA/FA on U(VI) adsorption was found at low pH, whereas a negative effect was observed at high pH. The presence of HA/FA enhanced the U(VI) adsorption at low pH values, but reduced U(VI) adsorption at high pH. The thermodynamic parameters (ΔH 0, ΔS 0, and ΔG 0) were also calculated from the temperature dependent adsorption isotherms, and the results suggested that the adsorption of U(VI) on Na-rectorite was a spontaneous and endothermic process.









Similar content being viewed by others
Change history
03 January 2020
Correction to: J Radioanal Nucl Chem (2011) 287:557���565
References
Hsyun SP, Cho YH, Hahn PS, Kim SJ (2001) J Radioanal Nucl Chem 250:55–62
Akyil S, Aslani MAA, Eral M (2003) J Radioanal Nucl Chem 256:45–51
Sylwester ER, Hudson EA, Allen PG (2000) Geochim Cosmochim Acta 64:2431–2438
Liao X, Lu Z, Xu D, Liu X, Shi B (2004) Environ Sci Technol 38:324–328
Sachs S, Bernhard G (2008) Chemosphere 72:1441–1447
Baik MH, Cho WJ, Hahn PS (2004) J Radioanal Nucl Chem 260:495–502
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) J Phys Chem B 113:860–864
Zhang G, Yang X, Liu Y, Jia Y, Yu G, Ouyang S (2004) J Colloid Interface Sci 278:265–269
Zhang G, Liu Y, Xie Y, Yang X (2005) Appl Clay Sci 29:15–21
Tan X, Chen C, Yu S, Wang X (2008) Appl Geochem 23:2767–2777
Yu S, Chen C, Chang P, Wang T, Lu S, Wang X (2008) Appl Clay Sci 38:219–226
Chang P, Yu S, Chen T, Ren A, Chen C, Wang X (2007) J Radioanal Nucl Chem 274:153–160
Wu W, Fan Q, Lu S, Niu S, Wang X (2006) Adsorpt Sci Technol 24:601–610
Hu J, Chen C, Sheng G, Li J, Chen Y, Wang X (2010) Radiochim Acta 98:1–9
Xu D, Chen C, Tan X, Jun H, Wang X (2007) Appl Geochem 22:2892–2906
Tan X, Wang X, Geckeis H, Rabung T (2008) Environ Sci Technol 42:6532–6537
Tan X, Fan Q, Wang X, Grambow B (2009) Environ Sci Technol 43:3115–3121
Tan X, Chang P, Fan Q, Zhou X, Yu SM, Wang S, Wang X (2008) Colloids Surf A 328:8–14
Du Q, Sun Z, Forsling W, Tang H (1997) J Colloid Interface Sci 187:221–231
Naveau A, Monteil-Rivera F, Dumonceau J, Catalette H, Simoni E (2006) J Colloid Interface Sci 293:27–35
Ordoñez-Regil E, Drot R, Simoni E (2003) J Colloid Interface Sci 263:391–399
Giustetto R, Xamena FXL, Ricchiardi G, Bordiga S, Damin A, Gobetto R, Chierotti MR (2005) J Phys Chem B 109:19360–19368
Ho YS, McKay G (2000) Water Res 34:735–742
Gorman-Lewis D, Burns PC, Fein JB (2008) J Chem Thermodyn 40:335–352
Gorman-Lewis D, Fein JB, Burns PC, Szymanowski JES, Converse J (2008) J Chem Thermodyn 40:980–990
Kowal-Fouchard A, Drot R, Simoni E, Ehrhardt JJ (2004) Environ Sci Technol 38:1399–1407
Fan Q, Shao D, Lu Y, Wang S, Wang X (2009) Chem Eng J 150:188–195
Ren X, Wang S, Yang S (2010) J Radioanal Nucl Chem 283:253–259
Montavon G, Markai S, Andres Y, Grambow B (2002) Environ Sci Technol 36:3303–3309
Takahashi Y, Minai Y, Ambe S, Makide Y, Ambe F (1999) Geochim Cosmochim Acta 63:815–836
Xu D, Wang X, Chen C, Zhou X, Tan X (2006) Radiochim Acta 94:429–434
Xu D, Shao D, Chen C, Ren AP, Wang X (2006) Radiochim Acta 94:97–102
Bhattacharyya KG, Gupta SS (2008) Colloid Surf. A 317:71–79
Sheng G, Shao D, Fan Q, Xu D, Chen Y, Wang X (2009) Radiochim Acta 97:621–630
Langmuir I (1918) J Am Chem Soc 40:1361–1403
Tan X, Wang X, Fang M, Chen C (2007) Colloid Surf. A 296:109–116
Ibrahim HA, El-Kamash AM, Hanafy M, Abdel-Monem NM (2008) Chem Eng J 144:67–74
Kilpatrick M, Baker L Jr, McKinney C Jr (1953) J Phys Chem 57:385–390
Shahwan T, Erten HN (2004) J Radioanal Nucl Chem 260:43–48
Donat R, Akdogan A, Erdem E, Cetisli H (2005) J Colloid Interface Sci 286:43–52
Ozcan A, Oncu E, Ozcan A (2006) Colloid Surf A 277:90–97
Khan AA, Singh RP (1987) Colloids Surf A 24:33–42
Li W, Pan G, Zhang MY, Zhao DY, Yang YH, Chen H, He GZ (2008) J Colloid Interface Sci 319:385–391
Chang P, Wang X, Yu S, Wang S (2007) Colloid Surf A 302:75–81
Shao D, Fan Q, Li J, Niu Z, Wu W, Chen Y, Wang X (2009) Microporous Mesoporous Mater 123:1–9
Acknowledgments
Financial supports from the National Natural Science Foundation of China (20907055; 20971126) and Knowledge Innovation Program of CAS are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, D., Yang, S., Chen, S. et al. Effect of pH, ionic strength and humic substances on the adsorption of Uranium (VI) onto Na-rectorite. J Radioanal Nucl Chem 287, 557–565 (2011). https://doi.org/10.1007/s10967-010-0846-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0846-4