Abstract
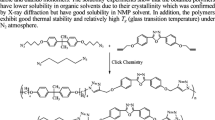
In this article, three new poly(1,2,3-triazolyl-benzenesulfonamide)s (4-6) were synthesized from the new molecule 4-azido-N-(prop-2ʹ-yn-1ʹ-yl)benzenesulfonamide (3). The synthesis and step-growth click polyaddition reaction of a new AB-type monomer containing terminal alkyne and azide groups were described. Copper (I)-catalyzed azide-alkyne cycloaddition (CuAAC) and Huisgen click polymerization were performed to prepare sulfonamide, a derivative of poly(1,2,3-triazole)s. The CuAAC polymerization regioselectively led to 1,4-disubstituted triazole ring and hence to a stereoregular polymer. In contrast, the thermal polymerization also produced 1,5-disubstituted triazole units. The structure of the new poly(1,2,3-triazolyl-benzenesulfonamide)s (4-6) obtained from (4-azido-N-(prop-2ʹ-yn-1ʹ-yl)benzenesulfonamide) were characterized by FTIR, 1H-, and 13C- NMR spectroscopy. The resulting polymers had weight-average molecular weights in the 7,700-58,650 range and were determined by Gel Permeation Chromatography (GPC). The resulting polymers 4, 5 and 6, lossing about 50% of their weights, showed a decomposition temperature of 406, 474, and 451 °C in a nitrogen atmosphere, respectively. The glass transition temperature of all polymers were at 128 °C. In addition, the surface morphology was studied via Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM/EDS). This paper is dedicated to the 100th Anniversary of the Republic of Türkiye.





Similar content being viewed by others
References
Gholami H, Yeganeh H, Gharibi R, Jalilian M, Sorayya M (2015) Catalyst free-click polymerization: a versatile method for the preparation of soybean oil based poly1,2,3-triazoles as coatings with efficient biocidal activity and excellent cytocompatibility. Polymer 62:94–108
Halay E, Ay E, Şalva E, Ay K, Karayıldırım T (2017) Syntheses of 1,2,3-triazole-bridged pyranose sugars with purine and pyrimidine nucleobases and evaluation of their anticancer potential. Nucleosides, Nucleotides Nucleic Acids 36:598–619
Bozorov K, Zhao J, Aisa HA (2019) 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: a recent overview. Bioorg Med Chem 27:3511–3531
Halay E, Ay E, Şalva E, Ay K, Karayıldırım T (2018) Synthesis of triazolylmethyl-linked nucleoside analogs via combination of azidofuranoses with propargylated nucleobases and study on their cytotoxicity. Chem Heterocycl Compd 54:158–166
Rani A, Singh G, Singh A, Maqbool U, Kaur G, Singh J (2020) CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery. RSC Adv 10:5610–5635
Öztürkoğlu G, Ay E, Alp H, Ay K (2021) 2-Amino-tiyazol Grubu İçeren 5-Florourasil Dimerinin Klik Tepkimesi Yoluyla Sentezi. J El-Cezeri 8:315–332
Zhao S, Liu J, Lv Z, Zhang G, Xu Z (2023) Recent updates on 1, 2, 3-triazole-containing hybrids with in vivo therapeutic potential against cancers: a mini-review. Eur J Med Chem 251:115254
De O, Torres NMP, Cardoso GDA, Silva H, De Freitas RP, Alves RB (2023) New purine-triazole hybrids as potential anti-breast cancer agents: synthesis, antiproliferative activity, and ADMET in silico study. Med Chem Res 1–16
Brunel D, Dumur F (2020) Recent advances in organic dyes and fluorophores comprising a 1, 2, 3-triazole moiety. New J Chem 44:3546–3561
Ahmed F, Xiong H (2021) Recent developments in 1,2,3-triazole-based chemosensors. Dyes Pigm 185:108905
Singh G, George N, Singh R, Singh G, Sushma, Kaur G, Singh H, Singh J (2023) Ion recognition by 1, 2, 3-triazole moieties synthesized via “click chemistry. Appl Organomet Chem 37:e6897
Taggert BI, Walker C, Chen D, Wille U (2021) Substituted 1,2,3-triazoles: a new class of nitrification inhibitors. Sci Rep 11:1–12
Huo J, Hu H, Zhang M, Hu X, Chen M, Chen D, Liu J, Xiao G, Wang Y, Wen Z (2017) A mini review of the synthesis of poly-1,2,3-triazole-based functional materials. RSC Adv 7:2281–2287
Bayrak F, Oral A, Ay K (2021) Synthesis and characterization of novel 1,2,3-triazole-bridged oxime polyurethanes obtained from an isomannide derivative. Maced J Chem Chem 40:75–87
Bayrak F, Ay E, Oral A, Karayıldırım T, Ay K (2022) Synthesis of 1,2,3-triazole group-containing isomannide-based aromatic new polyurethanes. Iran Polym J 31:413–423
Sykam K, Donempudi S, Basak P (2022) 1,2,3-Triazole rich polymers for flame retardant application: a review. J Appl Polym Sci 139:e52771
Ay K, Ispartaloğlu B, Halay E, Ay E, Yaşa İ, Karayıldırım T (2017) Synthesis and antimicrobial evaluation of sulfanilamide-and carbohydrate-derived 1,4-disubstitued-1,2,3-triazoles via click chemistry. Med Chem Res 26:1497–1505
Huisgen LR (1963) Kinetik und Mechanismus 1.3-Dipolarer Cycloadditionen. Angew Chem 75:742–754
Kolb HC, Finn M, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004–2021
Meldal M, Tornøe CW (2008) Cu-catalyzed azide-alkyne cycloaddition. Chem Rev 108:2952–3015
Tornøe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase:[1,2,3]-triazoles by regiospecific copper (I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67:3057–3064
Fernholm A (2022) The Nobel Prize In Chemistry. The Royal Swedish Academy of Science. https://www.nobelprize.org/prizes/chemistry/2022/summary/. [09.07.2023]
Kumar V, Lal K, Tittal RK (2023) The fate of heterogeneous catalysis & click chemistry for 1, 2, 3-triazoles: Nobel prize in chemistry 2022. Catal Commun 176:106629
Yamasaki S, Kamon Y, Xu L, Hashidzume A (2021) Synthesis of dense 1,2,3-triazole polymers soluble in common organic solvents. Polymers 13:1627
Huo J, Hu Z, Chen D, Luo S, Wang Z, Gao Y, Zhang M, Chen H (2017) Preparation and characterization of poly-1,2,3-triazole with chiral 2(5H)-furanone moiety as potential optical brightening agents. ACS Omega 2:5557–5564
Marrocchi A, Facchetti A, Lanari D, Santoro S, Vaccaro LJCS (2016) Click-chemistry approaches to π-conjugated polymers for organic electronics applications. Chem Sci 7:6298–6308
Bakhshi H, Yeganeh H, Mehdipour-Ataei S, Solouk A, Irani S (2013) Polyurethane coatings derived from 1,2,3-triazole-functionalized soybean oil-based polyols: studying their physical, mechanical, thermal, and biological properties. Macromolecules 46:7777–7788
Lai J, Yang F, Guo H, Jiao Z (2014) Novel effective dye sorbents: synthesis and properties of 1, 2, 3-triazole-modified thiacalix [4] arene polymers based on click chemistry. Iran Polym J 23:899–906
Johnson K, Lovinger J, Parker C, Baldwin M (1966) 1,3-Dipolar cycloaddition polymerization of 4‐azido‐1‐butyne. J Polym Sci B 4:977–979
Baldwin M, Johnson K, Lovinger J, Parker C (1967) 1,3-Dipolar cycloaddition polymerization of compounds containing both azido and acetylene groups. J Polym Sci B 5:803–806
Li Y, Zhou H, Wan EY, Huang L, Du F (2013) Synthesis and characterization of a new series of rigid polytriazole resins. Des Monomers Polym 16:556–563
Vikas M (2011) Book High Performance Polymers and Engineering Plastics. John Wiley & Sons.9781118016695, Canada
Al-Hujaj AFaK, Jassem HH, Al-Masoudi AM (2020) A click synthesis, molecular docking, cytotoxicity on breast cancer (MDA-MB 231) and anti-HIV activities of new 1,4-disubstituted-1,2,3-triazole thymine derivatives. Russ J Bioorg Chem 46:360–370
Cardillo P, Gigante L, Lunghi A, Fraleoni-Morgera A, Zanirato P (2008) Hazardous N-containing system: thermochemical and computational evaluation of the intrinsic molecular reactivity of some aryl azides and diazides. New J Chem 32:47–53
Xue L, Wan L, Hu Y, Shen X, Huang F, Du L (2006) Thermal stability of a novel polytriazole resin. Thermochim Acta 448:147–153
Beghdadi S, Miladi IA, Addis D, Romdhane HB, Bernard J, Drockenmuller E (2012) Synthesis and polymerization of C-vinyl-and N-vinyl-1, 2, 3-triazoles. Polym Chem 3:1680–1692
Acknowledgments
This study was financially supported by the Research Projects Coordination Office at Manisa Celal Bayar University (Project Number: 2022-026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ay, E. Synthesis of new poly(1,2,3-triazolyl-benzenesulfonamide)s via step-growth polymerization and characterization of their structures. J Polym Res 30, 360 (2023). https://doi.org/10.1007/s10965-023-03734-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03734-2