Abstract
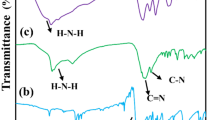
Due to the rarity of gold as a noble metal, efficient and selective Au (III) ions adsorption is critical for gold recovery. Since gold is thiophilic, in this paper, a new covalent thiourea organic polymer (CTOP) was prepared by introducing thiourea reactive groups into the covalent organic polymer of enaminone and applied to the adsorption of Au (III) ions in aqueous solution. The synthesis of the new material was confirmed by SEM, TEM, XRD, and XPS tests. Thanks to the abundant thiourea chelating sites in the CTOP, the total adsorption capacity of the material for Au (III) ions is up to 2372.7 mg/g. In addition, in mixed ionic solutions containing Au (III), Cu (II), Co (II), Cd (II), and Ni (II), it has strong selective adsorption performance for Au (III). It is worth mentioning that the CTOP material still maintains high adsorption performance after five adsorption cycles, which proves that the adsorption material has good reusability. The adsorption kinetics were investigated using pseudo-first-order and pseudo-second-order kinetic models, and the results showed that the adsorption was chemisorption. According to the experimental results of adsorption kinetics and thermodynamics, the adsorption process of CTOP for Au (III) ions conforms to the quasi-secondary adsorption model and the Langmuir adsorption model. We believe that the study of efficient adsorption of Au (III) ions by this new covalent thiourea organic polymer is of great significance for the development of gold recovery technology.










Similar content being viewed by others
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Huang X, Wang Y, Liao X, Shi B (2010) Adsorptive recovery of Au3+ from aqueous solutions using bayberry tannin-immobilized mesoporous silica. J Haz Mat 183:793–798. https://doi.org/10.1016/j.jhazmat.2010.07.096
Parajuli D, Adhikari CR, Kawakita H, Yamada S, Ohto K, Inoue K (2009) Chestnut pellicle for the recovery of gold. Bio-Tech 100:1000–1002. https://doi.org/10.1016/j.biortech.2008.06.058
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Haz Mat 158:228–256. https://doi.org/10.1016/j.jhazmat.2008.02.001
Pang S, Yung K (2014) Prerequisites for achieving gold adsorption by multiwalled carbon nanotubes in gold recovery. Chem Eng Sci 107:58–65. https://doi.org/10.1016/j.ces.2013.11.026
Hyun SP, Young JK (2018) A novel process of extracting precious metals from waste printed circuit boards: utilization of gold concentrate as a fluxing material. J Haz Mat 365:659–664. https://doi.org/10.1016/j.jhazmat.2018.11.051
Awadalla FT, Ritcey GM (1991) Recovery of gold from thiourea, thiocyanate, or thiosulfate solutions by reduction-precipitation with a stabilized form of sodium borohydride. Sep Sci Technol 26:1207–1228. https://doi.org/10.1080/01496399108050525
Shamsipur M, Ramezani M, Sadeghi M (2009) Preconcentration and determination of ultra-trace amounts of palladium in water samples by dispersive liquid-liquid microextraction and graphite furnace atomic absorption spectrometry. Microchim Acta 166:235–242. https://doi.org/10.1007/s00604-009-0186-7
Wang H, Bao C, Li F, Kong X, Xu J (2010) Preparation and application of 4-amino-4′-nitro azobenzene modified chitosan as a selective adsorbent for the determination of au (III) and pd(II). Microchim Acta 168:99–105. https://doi.org/10.1007/s00604-010-0355-8
Dwivedi AD, Dubey SP, Hokkanen S, Fallah RN, Sillanpää M (2014) Recovery of gold from aqueous solutions by taurine modified cellulose: an adsorptive–reduction pathway. Chem Eng J 255:97–106. https://doi.org/10.1016/j.cej.2014.06.017
Dong Z, Liu J, Yuan W, Yi Y, Zhao L (2016) Recovery of au (III) by radiation synthesized aminomethyl pyridine functionalized adsorbents based on cellulose. Chem Eng J 283:504–513. https://doi.org/10.1016/j.cej.2015.07.011
Doidge ED, Carson I, Tasker PA, Ellis RJ, Morrison CA, Love JB (2016) A simple primary amide for the selective recovery of gold from secondary resources. Angew Chem Int Ed 55:1–5. https://doi.org/10.1002/anie.201606113
Awual MR, Khaleque MA, Ferdows M, Chowdhury AMS, Yaita T (2013) Rapid recognition and recovery of gold (III) with functional ligand immobilized novel mesoporous adsorbent. Microchem J 110:591–598. https://doi.org/10.1016/j.microc.2013.07.010
Tauetsile PJ, Oraby EA, Eksteen JJ (2018) Adsorption behaviour of copper and gold glycinates in alkaline media onto activated carbon, part 1: Isotherms. Hydrometallurgy 178:202–208. https://doi.org/10.1016/j.hydromet.2018.04.015
Guo L, Liu C, Guo Z, Sun L, Liu J (2012) Preparation of polyvinyl chloride-based thiosemicarbazide resin and its selective adsorption of Ag(I) and au(III). J Disper Sci Technol 33:690–696. https://doi.org/10.1080/01932691.2011.579833
Shaheen HA, Marwani HM, Soliman EM (2015) Selective adsorption of gold ions from complex system using oxidized multi-walled carbon nanotubes. J Mol Liq 212:480–486. https://doi.org/10.1016/j.molliq.2015.09.040
Esmeralda VA, Elías RF, Isabel L, Roberto BG, Guillermo VM, Rene RM (2017) Gold recovery from very dilute solutions from a mine in closing process: Adsorption-desorption onto carbon materials. J Mol Liq 240:549–555. https://doi.org/10.1016/j.molliq.2017.05.069
Aydın A, İmamoğlu M, Gülfen M (2008) Separation and recovery of gold (III) from base metal ions using melamine–formaldehyde–thiourea chelating resin. J Appl Polym Sci 107:1201–1206. https://doi.org/10.1002/app.27178
Changmei S, Guanghua Z, Chunhua W, Rongjun Q, Ying Z, Quanyun G (2011) A resin with high adsorption selectivity for au (III): Preparation, characterization and adsorption properties. Chem Eeg J 172:713–720. https://doi.org/10.1016/j.cej.2011.06.040
Puthiaraj P, Lee Y, Zhang S, Ahn W (2016) Triazine-based covalent organic polymers: design, synthesis and applications in heterogeneous catalysis. J Mater Chem A 4:16288–16311. https://doi.org/10.1039/C6TA06089G
Arriagada R, García R (1997) Retention of aurocyanide and thiourea-gold complexes on activated carbons obtained from lignocellulosic materials. Hydrometallurgy 46:171–180. https://doi.org/10.1016/S0304-386X(97)00010-8
Xue D, Wang H, Liu Y, Shen P (2015) Multicolumn solid phase extraction with hybrid adsorbent and rapid determination of au, pd and pt in geological samples by GF-AAS. Min Eng 81:149–151. https://doi.org/10.1016/j.mineng.2015.07.025
Murakami H, Nishihama S, Yoshizuka K (2015) Separation and recovery of gold from waste LED using ion exchange method. Hydrometallurgy 157:194–198. https://doi.org/10.1016/j.hydromet.2015.08.014
An F, Li M, Wu R, Hu T, Gao J, Yuan Z (2017) Effective recovery of AuCl4– using D301 resin functionalized with ethylenediamine and thiourea. Hydrometallurgy 169:356–361. https://doi.org/10.1016/j.hydromet.2017.02.022
Mines PD, Thirion D, Uthuppu B, Hwang Y, Jakobsen MH, Andersen HR, Yavuz CT (2016) Covalent organic polymer functionalization of activated carbon surfaces through acyl chloride for environmental clean-up. Chem Eng J 309:766–771. https://doi.org/10.1016/j.cej.2016.10.085
Choi N, Kim B, Cho K, Lee S, Park C (2017) Microwave pretreatment for thiourea leaching for gold concentrate. Metals-Basel 7:404. https://doi.org/10.3390/met7100404
Rizki IN, Tanaka Y, Okibe N (2019) Thiourea bioleaching for gold recycling from e-waste. Waste Manag 84:158–165. https://doi.org/10.1039/C6TA06089G
Mironov IV, Makotchenko EV (2018) Transformations of the bis(thiourea)gold(I) complex in alkaline medium and interaction of thiourea with HAuCl4. Russ J Inorg Chem 63:1667–1672. https://doi.org/10.1134/S0036023618120161
Li A, Zheng NX, Yang T, Xie J, Li LJ, Tang KW, Zhou CS (2021) Highly selective enrichment of au using enaminone covalent organic polymers (COP). RSC Adv 11:29807–29815. https://doi.org/10.1039/D1RA03868K
Zhou S, Mo X, Zhu W, Xu W, Tang K, Lei Y (2020) Selective adsorption of au (III) with ultra-fast kinetics by a new metal-organic polymer. J Mol Liq 319:114125. https://doi.org/10.1016/j.molliq.2020.114125
Zhou S, Xu W, Hu C, Zhang P, Tang K (2020) Fast and effective recovery of au (III) from aqueous solution by a N-containing polymer. Chemosphere 260:127615. https://doi.org/10.1016/j.chemosphere.2020.127615
Wang C, Lin G, Zhao J, Wang S, Zhang L (2020) Enhancing au (III) adsorption capacity and selectivity via Engineering MOF with mercapto-1,3,4-thiadiazole. Chem Eng J 388:124221. https://doi.org/10.1016/j.cej.2020.124221
Zhao J, Wang C, Wang S, Zhang L, Zhang B (2019) Selective recovery of au(III) from wastewater by a recyclable magnetic Ni0.6Fe2.4O4 nanoparticels with mercaptothiadiazole: Interaction models and adsorption mechanisms. J Clean Prod 236:117605. https://doi.org/10.1016/j.jclepro.2019.117605
Zhao M, Huang Z, Wang S, Adhikari, Raj C (2020) Ultrahigh efficient and selective adsorption of au(III) from water by novel Chitosan-coated MoS2 biosorbents: performance and mechanisms. Chem Eng J 401:126006. https://doi.org/10.1016/j.cej.2020.126006
Zhang L, Zheng Q, Xiao S, Chen J, Jiang W, Cui W, Yang G, Liang R, Qiu J (2021) Covalent organic frameworks constructed by flexible alkyl amines for efficient gold recovery from leaching solution of e-waste. Chem Eng J 426:131865. https://doi.org/10.1016/j.cej.2021.131865
Ertan E, Gülfen M (2009) Separation of gold (III) ions from copper (II) and zinc (II) ions using thiourea-formaldehyde or urea-formaldehyde chelating resins. J Appl Polym Sci 111:2798–2805. https://doi.org/10.1002/app.29330
Liu J, Deng Z, Yu H, Wang L (2021) Ferrocene-based metal-organic framework for highly efficient recovery of gold from WEEE. Chem Eng J 410:128360. https://doi.org/10.1016/j.cej.2020.128360
Yang Y, Chen H, Wang X, Wang X, Li A, Xie J, Yi W, Li L, Zhou C (2023) A covalent organic polymer containing nitrogen and oxygen groups with high adsorption capacity and selectivity for gold ions under strongly acidic conditions. New J Chem 47:9575–9584. https://doi.org/10.1039/D3NJ00902E
Aydın A, İmamoğlu M, Gülfen M (2008) Separation and recovery of gold (III) from base metal ions using melamine–formaldehyde–thiourea chelating resin. J Appl Polym Sci 107:1201–1206. https://doi.org/10.1002/app.27178
Funding
The writers are very appreciative of the support from the National Natural Science Foundation of China (No. 51974122 and U20A20268), The Natural Science Foundation of Hunan Province (No. 2022JJ30279), and the Scientific Research Fund of Hunan Provincial Education Department (No. 20B268, 20B258, 20B261, and 19A209).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Tinglu Wang and Naixai Zheng. The first draft of the manuscript was written by Lijun Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Zheng, N., Li, A. et al. Synthesis of covalent thiourea organic polymer (CTOP) and adsorption of au (III) ions. J Polym Res 30, 279 (2023). https://doi.org/10.1007/s10965-023-03653-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03653-2