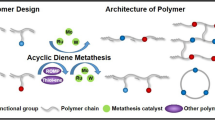
Abstract
This work aims to generate maleimides to polymers via Diels–Alder reaction and ring-opening metathesis polymerization (ROMP). Maleimides were converted to adducts through Diels–Alder reactions by reacting with cyclopentadiene or furan, then these adducts were generated to the corresponding polymers via ring-opening metathesis polymerization. The chemical structures of adducts were confirmed by 1H NMR, FTIR and elemental analysis. After investigating the polymerization behaviors and properties, we found that the adducts could be converted into polymers via ROMP and affording polymers exhibited excellent performance. For example, the glass transition temperature of PBMC is 252 °C, 5% and 10% weight loss temperatures are successively 381 °C and 475 °C, and the relative dielectric constant (Dk) and dissipation (Df) at 1 Hz and 1 MHz are respectively 3.41 and 0.006, 3.35 and 0.020. Moreover, the adducts can be used to enhance other ROMP-derived polymers as the comonomers. We believe these may help researchers design and explore novel maleimides polymers and copolymers with promising properties.






Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Bhattacharyya AS, Kumar S, Sharma A et al (2017) Metallization and APPJ treatment of bismaleimide. High Perform Polym 29:816–826. https://doi.org/10.1177/0954008316659123
Chong W, Lin L (2017) N-phenyl maleimide grafted MWNT/bismaleimide-allyl bisphenol A nanocomposites: Improved MWNT dispersion, resin reactivity and composite mechanical strength. Mater Lett 194:38–41. https://doi.org/10.1016/j.matlet.2017.02.014
Coope TS, Turkenburg DH, Fischer HR et al (2016) Novel Diels-Alder based self-healing epoxies for aerospace composites. Smart Mater Struct 25:084010. https://doi.org/10.1088/0964-1726/25/8/084010
Yu L, Yu Y, Shi J et al (2022) Synthesis of a novel hyperbranched polyimide for reinforcing toughness and insulating properties of bismaleimide resin. Polymers 14:4234. https://doi.org/10.3390/polym14194234
Ricarte RG, Tournilhac F, Leibler L (2019) Phase separation and self-assembly in vitrimers: hierarchical morphology of molten and semicrystalline polyethylene/dioxaborolane maleimide systems. Macromolecules 52:432–443. https://doi.org/10.1021/acs.macromol.8b02144
Yang F, Pan L, Ma Z et al (2020) Highly elastic, strong, and reprocessable cross-linked polyolefin elastomers enabled by boronic ester bonds. Polym Chem 11:3285–3295. https://doi.org/10.1039/d0py00235f
Belabbes A, Selva V, Foubelo F et al (2021) Synthesis of spiro{pyrrolidine-3,1 ’-pyrrolo 3,4-c pyrrole} basic framework by multicomponent 1,3-dipolar cycloaddition. Eur J Org Chem 2021:4229–4236. https://doi.org/10.1002/ejoc.202100646
Hiltebrandt K, Pauloehrl T, Blinco JP et al (2015) lambda-Orthogonal pericyclic macromolecular photoligation. Angew Chem Int Edit 54:2838–2843. https://doi.org/10.1002/anie.201410789
Lou L, Jiang L, Liu J et al (2007) Synthesis and characterization of optically active star-shaped poly (N-phenylmaleimide)s with a calixarene core. Polym Int 56:796–802. https://doi.org/10.1002/pi.2211
Klein R, Uebel F, Frey H (2015) Maleimide glycidyl ether: a bifunctional monomer for orthogonal cationic and radical polymerizations. Macromol Rapid Commun 36:1822–1828. https://doi.org/10.1002/marc.201500400
Nakagawa S, Taguchi M, Kimura A (2011) LET and dose rate effect on radiation-induced copolymerization of maleimide with styrene in 2-propanol solution. Radiat Phys Chem 80:1199–1202. https://doi.org/10.1016/j.radphyschem.2011.05.012
Yilmaz II, Arslan M, Sanyal A (2012) Design and synthesis of novel “Orthogonally” functionalizable maleimide-based styrenic copolymers. Macromol Rapid Commun 33:856–862. https://doi.org/10.1002/marc.201200036
Azechi M, Toyota N, Yamabuki K et al (2011) Anionic polymerization of N-substituted maleimide with achiral and chiral amines as an initiator. Polym Bull 67:631–640. https://doi.org/10.1007/s00289-010-0416-5
Xiao SJ, Wieland M, Brunner S (2005) Surface reactions of 4-aminothiophenol with heterobifunctional crosslinkers bearin both succinimidl ester and maleimide for biomolecular immobilization. J Colloid Interface Sci 290:172–183. https://doi.org/10.1016/j.jcis.2005.04.014
Bindu RL, Nair CPR, Ninan KN (2000) Phenolic resins bearing maleimide groups: Synthesis and characterization. J Polym Sci Pol Chem 38:641–652. https://doi.org/10.1002/(sici)1099-0518(20000201)38:3%3c641::Aid-pola28%3e3.0.Co;2-z
Bindu RL, Nair CPR, Ninan KN (2001) Phenolic resins with phenyl maleimide functions: Thermal characteristics and laminate composite properties. J Appl Polym Sci 80:1664–1674. https://doi.org/10.1002/app.1261
Parker S, Reit R, Abitz H et al (2016) High-T-g Thiol-Click Thermoset Networks via the Thiol-Maleimide Michael Addition. Macromol Rapid Commun 37:1027–1032. https://doi.org/10.1002/marc.201600033
Gouri C, Nair CPR, Ramaswamy R et al (2002) Thermal decomposition characteristics of Alder-ene adduct of diallyl bisphenol A novolac with bismaleimide: effect of stoichiometry, novolac molar mass and bismaleimide structure. Eur Polym J 38:503–510. https://doi.org/10.1016/s0014-3057(01)00197-5
Nishimori K, Tenjimbayashi M, Naito M et al (2020) Alternating copolymers of vinyl catechol or vinyl phenol with alkyl maleimide for adhesive and water-repellent coating materials. ACS Appl Polym Mater 2:4604–4612. https://doi.org/10.1021/acsapm.0c00682
Chaisuwan T, Ishida H (2006) High-performance maleimide and nitrile-functionalized benzoxazines with good processibility for advanced composites applications. J Appl Polym Sci 101:548–558. https://doi.org/10.1002/app.23509
Ishida H, Ohba S (2005) Synthesis and characterization of maleimide and norbornene functionalized benzoxazines. Polymer 46:5588–5595. https://doi.org/10.1016/j.polymer.2005.04.080
Berto P, Mehats J, Wirotius A-L et al (2022) Reprocessable covalent elastomeric networks from functionalized 1,4-cis-polyisoprene and -polybutadiene. Macromolecules 55:4557–4567. https://doi.org/10.1021/acs.macromol.1c02156
Berto P, Pointet A, Le Coz C et al (2018) Recyclable telechelic cross-linked polybutadiene based on reversible diels-alder chemistry. Macromolecules 51:651–659. https://doi.org/10.1021/acs.macromol.7b02220
Dispinar T, Sanyal R, Sanyal A (2007) A Diels-Alder/Retro diels-alder strategy to synthesize polymers bearing maleimide side chains. J Polym Sci Pol Chem 45:4545–4551. https://doi.org/10.1002/pola.22299
Oz Y, Sanyal A (2018) The Taming of the Maleimide: Fabrication of Maleimide-Containing “Clickable” polymeric materials. Chem Rec 18:570–586. https://doi.org/10.1002/tcr.201700060
Zhou W, Zhang H, Chen F (2018) Modified lignin: Preparation and use in reversible gel via Diels-Alder reaction. Int J Biol Macromol 107:790–795. https://doi.org/10.1016/j.ijbiomac.2017.09.052
Froidevaux V, Borne M, Laborbe E et al (2015) Study of the Diels-Alder and retro-Diels-Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv 5:37742–37754. https://doi.org/10.1039/c5ra01185j
Gandini A (2005) The application of the Diels-Alder reaction to polymer syntheses based on furan/maleimide reversible couplings. Polímeros 15:95–101. https://doi.org/10.1590/s0104-14282005000200007
Gandini A (2013) The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog Polym Sci 38:1–29. https://doi.org/10.1016/j.progpolymsci.2012.04.002
Yameen B, Rodriguez-Emmenegger C, Preuss CM et al (2013) A facile avenue to conductive polymer brushes via cyclopentadiene-maleimide Diels-Alder ligation. Chem Commun 49:8623–8625. https://doi.org/10.1039/c3cc44683b
Zhou D, Huang H, Wang Y et al (2020) Design and synthesis of an amide-containing crosslinked network based on Diels-Alder chemistry for fully recyclable aramid fabric reinforced composites. Compos Sci Technol 197:108280. https://doi.org/10.1016/j.compscitech.2020.108280
Cioc RC, Crockatt M, Waal JC et al (2022) The Interplay between Kinetics and Thermodynamics in Furan Diels-Alder Chemistry for Sustainable Chemicals Production. Angew Chem Int Edit 61:e202114720. https://doi.org/10.1002/anie.202114720
Luo K-J, Huang L-B, Wang Y et al (2020) Tailoring the properties of Diels-Alder reaction crosslinked high-performance thermosets by different bismaleimides. Chin J Polym Sci 38:268–277. https://doi.org/10.1007/s10118-019-2328-7
Hoveyda AH, Zhugralin AR (2007) The remarkable metal-catalysed olefin metathesis reaction. Nature 450:243–251. https://doi.org/10.1038/nature06351
Church DC, Takiguchi L, Pokorski JK (2020) Optimization of ring-opening metathesis polymerization (ROMP) under physiologically relevant conditions. Polym Chem 11:4492–4499. https://doi.org/10.1039/d0py00716a
Yasir M, Liu P, Tennie IK et al (2019) Catalytic living ring-opening metathesis polymerization with Grubbs’ second- and third-generation catalysts. Nat Chem 11:488–494. https://doi.org/10.1038/s41557-019-0239-4
Blosch SE, Scannelli SJ, Alaboalirat M et al (2022) Complex polymer architectures using ring-opening metathesis polymerization: synthesis, applications, and practical considerations. Macromolecules 55:4200–4227. https://doi.org/10.1021/acs.macromol.2c00338
Chen K, Han W, Hu X et al (2022) Microreactor-based chemo-enzymatic ROP-ROMP platform for continuous flow synthesis of bottlebrush polymers. Chem Eng J 437:135284. https://doi.org/10.1016/j.cej.2022.135284
Naguib M, Nixon KL, Keddie DJ (2022) Effect of radical copolymerization of the (oxa)norbornene end-group of RAFT-prepared macromonomers on bottlebrush copolymer synthesis via ROMP. Polym Chem 13:1401–1410. https://doi.org/10.1039/d1py01599k
Conrad RM, Grubbs RH (2009) Tunable, temperature-responsive polynorbornenes with side chains based on an elastin peptide sequence. Angew Chem Int Edit 48:8328–8330. https://doi.org/10.1002/anie.200903888
Parry AL, Bomans PHH, Holder SJ et al (2008) Cryo electron tomography reveals confined complex morphologies of tripeptide-containing amphiphilic double-comb diblock copolymers. Angew Chem Int Edit 47:8859–8862. https://doi.org/10.1002/anie.200802834
Alvaradejo GG, Nguyen HVT, Harvey P et al (2019) Polyoxazoline-based bottlebrush and brush-arm star polymers via ROMP: Syntheses and applications as organic radical contrast agents. ACS Macro Lett 8:473–478. https://doi.org/10.1021/acsmacrolett.9b00016
Golder MR, Nguyen HVT, Oldenhuis NJ et al (2018) Brush-first and ROMP-out with functional (macro)monomers: method development, structural investigations, and applications of an expanded brush-arm star polymer platform. Macromolecules 51:9861–9870. https://doi.org/10.1021/acs.macromol.8b01966
Zhang T, Sui X, Gutekunst WR (2021) Convergent synthesis of branched metathesis polymers with enyne reagents. Macromolecules 54:8435–8442. https://doi.org/10.1021/acs.macromol.1c01051
Hore S, Singh A, De S et al (2022) Polyarylquinone synthesis by relayed dehydrogenative 2+2+2 cCycloaddition. ACS Catal 12:6227–6237. https://doi.org/10.1021/acscatal.2c00175
Zhang L, Huangfu F, Li W et al (2021) Rapid synthesis of diol homolog-based thermosets with tunable properties via ring-opening metathesis polymerization. Mater Adv 2:3671–3676. https://doi.org/10.1039/d1ma00210d
Huangfu F, Li W, Yang Z et al (2022) Bulk ring-opening metathesis copolymerization of dicyclopentadiene and 5-ethylidene-2-norbornene: mixing rules, polymerization behaviors and properties. J Polym Res 29:420. https://doi.org/10.1007/s10965-022-03268-z
Li W, Zhan Q, Yang P (2023) Facile approach for the synthesis of performance-advantaged degradable bio-based thermoset via ring-opening metathesis polymerization from epoxidized soybean oil. ACS Sustain Chem Eng 11:1200–1206. https://doi.org/10.1021/acssuschemeng.2c06787
Peng W, Chen X, Wang J (2021) Study on the curing behavior of polythiol/phenolic/epoxy resin and the mechanical and thermal properties of the composites. Mater Res Express 8:055302. https://doi.org/10.1088/2053-1591/abeb4a
Adjaoud A, Puchot L, Verge P (2022) High-Tg and degradable isosorbide-based polybenzoxazine vitrimer. ACS Sustain Chem Eng 10:594–602. https://doi.org/10.1021/acssuschemeng.1c07093
Zhang S, Ran Q, Fu Q et al (2018) Preparation of transparent and flexible shape memory polybenzoxazine film through chemical structure manipulation and hydrogen bonding control. Macromolecules 51:6561–6570. https://doi.org/10.1021/acs.macromol.8b01671
Zhang S, Li Q, Ye J et al (2022) Probing the copolymerization of alkynyl and cyano groups using monocyclic benzoxazine as model compound. Polymer 252:124932. https://doi.org/10.1016/j.polymer.2022.124932
Yang J, He X, Wang H et al (2020) High-toughness, environment-friendly solid epoxy resins: Preparation, mechanical performance, curing behavior, and thermal properties. J Appl Polym Sci 137:48596. https://doi.org/10.1002/app.48596
Yin R, Cheng H, Hong C et al (2017) Synthesis and characterization of novel phenolic resin/silicone hybrid aerogel composites with enhanced thermal, mechanical and ablative properties. Compos Part A Appl S 101:500–510. https://doi.org/10.1016/j.compositesa.2017.07.012
Rao Y, Ogitani S, Kohl P et al (2002) Novel polymer-ceramic nanocomposite based on high dielectric constant epoxy formula for embedded capacitor application. J Appl Polym Sci 83:1084–1090. https://doi.org/10.1002/app.10082
Hamerton I, Howlin BJ, Mitchell AL et al (2012) Systematic examination of thermal, mechanical and dielectrical properties of aromatic polybenzoxazines. React Funct Polym 72:736–744. https://doi.org/10.1016/j.reactfunctpolym.2012.07.001
Acknowledgements
The authors acknowledge support from the Guangdong SHENGYI Technology Limited Corporation for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This research does not include experiments involving human tissue and does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
The manuscript has not been published elsewhere and that it has not been submitted simultaneously for publication elsewhere.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Zhan, Q. & Yang, P. Synthesis of poly(maleimide)s with promising performance via Diels–Alder reaction and ring-opening metathesis polymerization. J Polym Res 30, 127 (2023). https://doi.org/10.1007/s10965-023-03503-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03503-1