Abstract
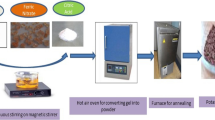
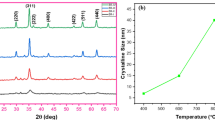
Hybrid iron-containing nanocomposite materials based on polyphenoxazine (PPOA) were synthesized for the first time via chemical transformations of PPOA in the presence of iron(III) salt (FeCl3·6H2O, Fe(NO3)3·6H2O or Fe(CH3COCH=C(CH3)O)3) in an inert atmosphere under IR radiation in the temperature range of 350–600 °С. The developed hybrid nanocomposites were characterized by means of FTIR spectroscopy, X-ray diffraction (XRD), transmission (TEM) and scanning (SEM) electron microscopy, X-Ray fluorescence spectroscopy (XRF), Inductively coupled plasma atomic emission spectroscopy (ICP-AES), atomic absorption spectrometry (AAS), elemental analysis, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and magnetometry. The dependence of phase composition, magnetic and thermal properties of hybrid nanocomposites, as well as the size and shape of nanoparticles on synthesis temperature, Fe concentration and the nature of iron(III) salt was demonstrated.
















Similar content being viewed by others
References
Godovsky DY (2000) Device applications of polymer-nanocomposites. Adv Polym Sci 153:163–205
Karpacheva GP (2016) Hybrid magnetic nanocomposites including polyconjugated polymers. Polym Sci 58:131–146
Yang С, Du J, Peng Q, Qiao R, Chen W, Xu C, Shuai Z, Gao M (2009) Polyaniline/Fe3O4 nanoparticle composite: synthesis and reaction mechanism. J Phys Chem B 113:5052–5058
Xiao Q, Tan X, Ji L, Xue J (2007) Preparation and characterization of polyaniline/nano-Fe3O4 composites via a novel Pickering emulsion route. Synth Met 157:784–791
Araújo ACV, Oliveira RJ, Alves Jr S, Rodrigues AR, Machado FLA, Cabral FAO, Azevedo WM (2010) Synthesis, characterization and magnetic properties of polyaniline-magnetite nanocomposites. Synth Met 160:685–690
Qiu G, Wang Q, Nie M (2006) Polyaniline/Fe3O4 magnetic nanocomposite prepared by ultrasonic irradiation. J Appl Polym Sci 102:2107–2111
Khan A, Aldwayan AS, Alhoshan M, Alsalhi M (2010) Synthesis by in situ chemical oxidative polymerization and characterization of polyaniline/iron oxide nanoparticle composite. Polym Int 59:1690–1694
Umare SS, Shambharkar BH (2013) Synthesis, characterization, and corrosion inhibition study of polyaniline-α-Fe2O3 nanocomposite. J Appl Polym Sci 127:3349–3355
Mallikarjuna NN, Manohar SK, Kulkarni PV, Venkataraman A, Aminabhavi TM (2005) Novel high dielectric constant nanocomposites of polyaniline dispersed with γ-Fe2O3 nanoparticles. J Appl Polym Sci 97:1868–1874
Bhaumik M, Leswifi TY, Maity A, Shrinivasu VV, Onyango MS (2011) Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic nanocomposite. J Hasardous Mater 186:150–159
Jokar M, Foroutani R, Safaralizadeh MH, Farhadi K (2014) Synthesis and characterization of polyaniline/Fe3O4 magnetic nanocomposite as practical approach for fluoride removal process. Ann Res Rev Biol 4:3262–3273
Shambharkar BH, Umare SS (2010) Production and characterization of polyaniline/Co3O4 nanocomposite as a cathode of Zn–polyaniline battery. Mater Sci Eng B 175:120–128
Sen T, Shimpi NG, Mishra S, Sharma R (2014) Polyaniline/γ-Fe2O3 nanocomposite for room temperature LPG sensing. Sensors Actuators B 190:120–126
Rezvani M, Asgharinezhad AA, Ebrahimzadeh H, Shekari N (2014) A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium (VI) ions. Microchim Acta 181:1887–1895
Chen A, Wang H, Zhao B, Wang J, Li X (2004) Preparation and characterization of Fe3O4/polypyrrole(PPy) composites. Acta Mater Compos Sin 21:157–160
Jacobo SE, Aphesteguy JC, Lopez Anton R, Schegoleva NN, Kurlyandskaya GV (2007) Influence of the preparation procedure on the properties of polyaniline based magnetic composites. Eur Polym J 43:1333–1346
Aphesteguy JC, Jacobo SE (2007) Synthesis of a soluble polyaniline–ferrite composite: magnetic and electric properties. J Mater Sci 42:7062–7068
Dzhardimalieva GI, Uflyand IE (2018) Preparation of metal-polymer nanocomposites by chemical reduction of metal ions: functions of polymer matrices. J Polym Res 25(255). https://doi.org/10.1007/s10965-018-1646-8
Karpacheva GP, Ozkan SZh (2011) RU Patent for the invention of "Dispersed nanocomposite magnetic material and method of its obtaining". № 2426188
Eremeev IS, Ozkan SZ, Karpacheva GP, Bondarenko GN (2014) Hybrid dispersed magnetic nanomaterial based on polydiphenylamine-2-carbonic acid and Fe3O4. Nanotechnologies in Russia 9:38–44
Karpacheva GP, Ozkan SZ, Eremeev IS, Bondarenko GN, Dzidziguri EL, Chernavskii PA (2014) Synthesis of hybrid magnetic nanomaterial based on polydiphenylamine-2-carboxylic acid and Fe3O4 in the interfacial process. Eur Chem Bull 3:1001–1007
Ozkan SZ, Karpacheva GP, Dzidziguri EL, Chernavskii PA, Bondarenko GN, Pankina GV (2017) Formation features of hybrid magnetic materials based on polyphenoxazine and magnetite nanoparticles. J Res Updates Polym Sci 5:137–148
Ozkan SZh, Karpacheva GP (2017) RU patent for the invention of "Metal-polymer nanocomposite magnetic material based on poly-3-amine-7-methylamine-2-methylphenazine and nanoparticles Fe3O4 and method of its production". № 2637333 C2
Ozkan SZ, Karpacheva GP, Chernavskii PA, Dzidziguri EL, Bondarenko GN, Pankina GV (2018) A hybrid material based on poly-3-amine-7-methylamine-2-methylphenazine and magnetite nanoparticles. Nanotechnologies in Russia 13:122–129
Ozkan SZh, Karpacheva GP (2017) RU patent for the invention of "Nanocomposite magnetic material based on poly-3-amine-7-methylamine-2-methylphenazine and Fe3O4 nanoparticles immobilized on single-walled carbon nanotubes and method of its production". № 2635254 С2
Ozkan SZ, Karpacheva GP, Chernavskii PA, Dzidziguri EL, Bondarenko GN, Pankina GV (2018) Hybrid materials based on poly-3-amine-7-methylamine-2-methylphenazine and magnetite nanoparticles immobilized on single-walled carbon nanotubes. Polymers 10:544
Aphesteguy JC, Jacobo SE (2004) Composite of polyaniline containing iron oxides. Physica B 354:224–227
Wan M, Li J (1998) Synthesis and electrical–magnetic properties of polyaniline composites. J Polym Sci A Polym Chem 36:2799–2805
Zhang Z, Wan M (2003) Nanostructures of polyaniline composites containing nano-magnet. Synth Met 132:205–212
Karpacheva G, Ozkan S (2013) Polymer-metal hybrid structures based on polydiphenylamine and Co nanoparticles. Procedia Mater Sci 2:52–59
Ozkan SZ, Dzidziguri EL, Chernavskii PA, Karpacheva GP, Efimov MN, Bondarenko GN (2013) Metal-polymer nanocomposites based on polydiphenylamine and cobalt nanoparticles. Nanotechnologies in Russia 8:452–460
Ozkan SZ, Dzidziguri EL, Karpacheva GP, Chernavskii PA, Efimov MN, Bondarenko GN (2015) A magnetic metal-polymer nanocomposite material based on polydiphenylamine and Fe3O4 nanoparticles. Russ Chem Bull 64:196–201
Karpacheva GP, Ozkan SZ, Dzidziguri EL, Chernavskii PA, Eremeev IS, Efimov MN, Ivantsov MI, Bondarenko GN (2015) Hybrid metal-polymer nanocomposites based on polyphenoxazine and cobalt nanoparticles. Eur Chem Bull 4:135–141
Karpacheva GP, Ozkan SZh (2016) RU patent for the invention of "Polymer dispersed magnetic material and method for production thereof". № 2601005
Ozkan SZ, Karpacheva GP, Dzidziguri EL, Chernavskii PA, Bondarenko GN, Efimov MN, Pankina GV (2017) One step synthesis of hybrid magnetic material based on polyphenoxazine and bimetallic Co-Fe nanoparticles. Polym Bull 74:3043–3060
Gubin SP, Koksharov YA, Khomutov GB, Yurkov GY (2005) Magnetic nanoparticles: preparation, structure and properties. Russ Chem Rev 74:489–520
Chernavskii PA, Pankina GV, Lunin VV (2011) Magnetometric methods of investigation of supported catalysts. Russ Chem Rev 80:579–604
Ozkan SZ, Karpacheva GP, Bondarenko GN (2011) Polymers of phenoxazine: synthesis, structure. Russ Chem Bull 60:1651–1656
Zemtsov LM, Karpacheva GP, Efimov MN, Muratov DG, Bagdasarova KA (2006) Carbon nanostructures based on IR-pyrolyzed polyacrylonitrile. Polym Sci A 48:633–637
Dzidziguri EL (2009) Dimensional characteristics of nanopowders. Nanotechnologies in Russia 4:857–870
Soloveva AY, Ioni YV, Gubin SP (2016) Synthesis of Fe3O4 nanoparticles on the surface of graphene. Mendeleev Comm 26:38–39
Cornell RM, Schwertmann U (1996) The iron oxides: structure, properties, reactions, occurrences and uses. (VCH, New York)
Gubin SP (2000) What is nanoparticle? Development trends for nanochemistry and nanotechnology. Ross Khim Zh 44:23–31
Acknowledgments
This work was carried out within the State Program of TIPS RAS. The equipment from the collective exploitation center “New petrochemical processes, polymer composites and adhesives” was used.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozkan, S.Z., Karpacheva, G.P., Leont’evna Dzidziguri, E. et al. Iron-containing magnetic nanocomposites based on polyphenoxazine. J Polym Res 26, 176 (2019). https://doi.org/10.1007/s10965-019-1843-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1843-0