Abstract
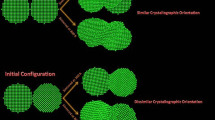
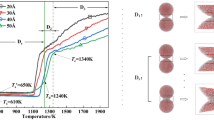
The sintering processes of many TiO\(_{2}\) nanoparticles in chains of both solid and liquid phases have been studied in detail via molecular dynamics simulation. For the liquid phase, a modified correlation for the characteristic sintering time of multi-particle chains is obtained by including a correction factor of \((N/2)^{1/3}\), where N is the number of primary particles. The temperature rise during sintering is found to be linearly proportional to \((1-N^{-1/3})\). Moreover, this study provides a way to calculate the surface energy of nanoparticles of small diameters in liquid phase, which is experimentally unattainable. For the solid phase, sintering induced nucleation is observed for \(N \ge 4\) cases both at \(T_{0 }= 1220\) and 960 K with a sharp increase in the temperature and a decrease in the potential energy. The formation of rutile from nucleation of many solid amorphous particles through sintering is observed for the first time.







Similar content being viewed by others
References
Goldstein, A.N., Echer, C.M., Alivisatos, A.P.: Melting in semiconductor nanocrystals. Science 256, 1425–1427 (1992)
Colvin, V.L., Alivisatos, A.P., Tobin, J.G.: Valence-band photoemission from a quantum-dot system. Phys. Rev. Lett. 66, 2786 (1991)
Yetter, R.A., Risha, G.A., Son, S.F.: Metal particle combustion and nanotechnology. Proc. Combust. Inst. 32, 1819–1838 (2009)
Kung, M.C., Davis, R.J., Kung, H.H.: Understanding Au-catalyzed low-temperature CO oxidation. J. Phys. Chem. C 111, 11767–11775 (2007)
Mueller, R., Mädler, L., Pratsinis, S.E.: Nanoparticle synthesis at high production rates by flame spray pyrolysis. Chem. Eng. Sci. 58, 1969–1976 (2003)
Swihart, M.T.: Vapor-phase synthesis of nanoparticles. Curr. Opin. Colloid Interface Sci. 8, 127–133 (2003)
Wang, J., Li, S., Yan, W., Tse, S.D., Yao, Q.: Synthesis of TiO\(_{2}\) nanoparticles by premixed stagnation swirl flames. Proc. Combust. Inst. 33, 1925–1932 (2011)
Pratsinis, S.E.: Flame aerosol synthesis of ceramic powders. Proc. Energy Combust. Sci. 24, 197–219 (1998)
Zachariah, M.R., Carrier, M.J.: Molecular dynamics computation of gas-phase nanoparticle sintering: a comparison with phenomenological models. J. Aerosol Sci. 30, 1139–1151 (1999)
Koch, W., Friedlander, S.K.: The effect of particle coalescence on the surface area of a coagulating aerosol. J. Colloid Interface Sci. 140, 419–427 (1990)
Frenkel, J.: On the theory of electric contacts between metallic bodies. J. Phys. 9, 385 (1945)
Pokluda, O., Bellehumeur, C.T., Vlachopoulos, J.: Modification of Frenkel’s model for sintering. AlChE J. 43, 3253–3256 (1997)
Friedlander, S.K., Wu, M.K.: Linear rate law for the decay of the excess surface-area of a coalescing solid particle. Phys. Rev. B 49, 3622–3624 (1994)
Martínez-Herrera, J.I., Derby, J.J.: Viscous sintering of spherical particles via finite element analysis. J. Am. Ceram. Soc. 78, 645–649 (1995)
Kirchhof, M.J., Schmid, H.J., Peukert, W.: Three-dimensional simulation of viscous-flow agglomerate sintering. Phys. Rev. E 80, 026319 (2009)
Eggersdorfer, M.L., Kadau, D., Herrmann, H.J., Pratsinis, S.E.: Multiparticle sintering dynamics: from fractal-like aggregates to compact structures. Langmuir 27, 6358–6367 (2011)
Eggersdorfer, M.L., Pratsinis, S.E.: Restructuring of aggregates and their primary particle size distribution during sintering. AIChE J. 59, 1118–1126 (2013)
Buesser, B., Grohn, A.J., Pratsinis, S.E.: Sintering rate and mechanism of TiO\(_{2}\) nanoparticles by molecular dynamics. J. Phys. Chem. C 115, 11030–11035 (2011)
Koparde, V.N., Cummings, P.T.: Molecular dynamics simulation of titanium dioxide nanoparticle sintering. J. Phys. Chem. B 109, 24280–24287 (2005)
Zhu, H.: Sintering processes of two nanoparticles: a study by molecular dynamics simulations. Philos. Mag. Lett. 73, 27–33 (1996)
Hawa, T., Zachariah, M.R.: Molecular dynamics simulation and continuum modeling of straight-chain aggregate sintering: development of a phenomenological scaling law. Phys. Rev. B 76, 054109 (2007)
Hawa, T., Zachariah, M.R.: Development of a phenomenological scaling law for fractal aggregate sintering from molecular dynamics simulation. J. Aerosol Sci. 38, 793–806 (2007)
Matsui, M., Akaogi, M.: Molecular dynamics simulation of the structural and physical properties of the four polymorphs of TiO\(_{2}\). Mol. Simul. 6, 239–244 (1991)
Buckingham, R.A.: The classical equation of state of gaseous helium, neon and argon. R. Soc. Lond. Proc. Ser. A Math. Phys. Eng. Sci. 168, 264–283 (1938)
Traylor, J.G., Smith, H.G., Nicklow, R.M., Wilkinson, M.K.: Lattice dynamics of rutile. Phys. Rev. B 3, 3457 (1971)
Plimpton, S.: Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995)
Koparde, V.N., Cummings, P.T.: Sintering of titanium dioxide nanoparticles: a comparison between molecular dynamics and phenomenological modeling. J. Nanoparticle Res. 10, 1169–1182 (2008)
Kazakov, A.V., Shpiro, E.S., Voskoboinikov, T.V.: Application of Debye function analysis to particle size and shape determination in Ir/SiO\(_{2}\) catalysts. J. Phys. Chem. 99, 8323–8327 (1995)
Koparde, V.N., Cummings, P.T.: Phase transformations during sintering of titania nanoparticles. ACS Nano 2, 1620–1624 (2008)
Cromer, D.T., Mann, J.B.: X-ray scattering factors computed from numerical Hartree-Fock wave functions. Acta Cryst. Sect. A 24, 321–324 (1968)
Buffat, P., Borel, J.P.: Size effect on the melting temperature of gold particles. Phys. Rev. A 13, 2287–2298 (1976)
Zhang, Y., Li, S., Yan, W., Stephen, D.T.: Effect of size-dependent grain structures on the dynamics of nanoparticle coalescence. J. Appl. Phys. 111, 124321 (2012)
Li, Y., Ishigaki, T.: Thermodynamic analysis of nucleation of anatase and rutile from TiO\(_{2}\) melt. J. Cryst. Growth 242, 511–516 (2002)
Dingwell, D.B.: The density of titanium(IV) oxide liquid. J. Am. Ceram. Soc. 74, 2718–2719 (1991)
Barnard, A.S., Zapol, P., Curtiss, L.A.: Modeling the morphology and phase stability of TiO\(_{2}\) nanocrystals in water. J. Chem. Theory Comput. 1, 107–116 (2005)
Naicker, P.K., Cummings, P.T., Zhang, H., Banfield, J.F.: Characterization of titanium dioxide nanoparticles using molecular dynamics simulations. J. Phys. Chem. B 109, 15243–15249 (2005)
Zhang, H., Peng, S., Long, X., et al.: Wall-induced phase transition controlled by layering freezing. Phys. Rev. E 89, 032412 (2014)
Wang, C., Deng, Z.X., Li, Y.: The synthesis of nanocrystalline anatase and rutile titania in mixed organic media. Inorg. Chem. 40, 5210–5214 (2001)
Zhang, H., Chen, B., Banfield, J.F.: The size dependence of the surface free energy of titania nanocrystals. PCCP 11, 2553–2558 (2009)
Zhao, B., Uchikawa, K., McCormick, J.R., Ni, C.Y., Chen, J.G., Wang, H.: Ultrafine anatase TiO\(_{2}\) nanoparticles produced in premixed ethylene stagnation flames. Proc. Combust. Inst. 30, 2569–2576 (2005)
Acknowledgments
Support from the Major Project of the National Science Foundation of China (Grant No. 51390493) and the Center for Combustion Energy at Tsinghua University is gratefully acknowledged. The simulations were partly performed on the Tsinghua High-Performance Parallel Computer supported by the Tsinghua National Laboratory for Information Science and Technology and partly on ARCHER funded under the EPSRC Project “UK Consortium on Mesoscale Engineering Sciences (UKCOMES)” (Grant No. EP/L00030X/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, Q., Luo, K.H. Molecular Dynamics Simulation of Sintering Dynamics of Many TiO\(_{2}\) Nanoparticles. J Stat Phys 160, 1696–1708 (2015). https://doi.org/10.1007/s10955-015-1304-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-015-1304-z