Abstract
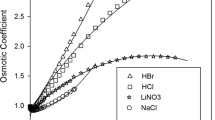
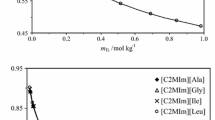
An Electrolyte non-random-UNIQUAC (NR-UNIQUAC) local composition model is developed for calculation of the excess Gibbs energy and activity coefficients for binary and the multicomponent electrolyte solutions. A new expression for the energies of the reference cells in the random state is used and a modified version of the UNIQUAC model is provided. The high efficiency of the presented model is demonstrated through the correlation of the available experimental data for various electrolyte solutions. Obtained results are compared with those of Electrolyte-UNIQUAC-NRF model. The correlation of data was carried out using two different approaches, the single system correlation and the global optimization of the interaction parameters. In the single fitting approach, two adjustable binary interaction parameters of the model are regressed using the experimental mean activity coefficient data of binary solutions. In the global approach, the anion-water and cation–anion interaction energy parameters of different ions are calculated via simultaneous correlation of the mean activity coefficient data of the forty-nine binary electrolyte solutions. In both approaches, the adjustable parameters are calculated in a wide range of electrolyte concentrations and at different temperatures, so that these parameters can be used to predict the osmotic coefficient data of many binary systems. Moreover, the solubility and osmotic coefficient data of ternary electrolyte solutions were predicted using the previously obtained binary parameters. Using the present model, the predicted data for the binary and multicomponent electrolyte solutions are in good agreement with the experimental data. When compared to Electrolyte-UNIQUAC-NRF model, the present model is more accurate. Additionally, the presence of salt–salt interaction parameters in the electrolyte-NR-UNIQUAC model can further improve its efficiency when experimental data for a ternary system is available.
















Similar content being viewed by others
References
Debye, P., Hückel, E.: De la theorie des electrolytes. I. Abaissement du point de congelation et phenomenes associes. Phys. Z. 24, 185–206 (1923)
Pitzer, K.S.: Electrolytes. From dilute solutions to fused salts. J. Am. Chem. Soc. 102, 2902–2906 (1980)
Guggenheim, E.A.: The specific thermodynamic properties of aqueous solutions of strong electrolytes. Philos. Mag. (Ser. 7) 19, 588–643 (1935)
Guggenheim, E.A., Turgeon, J.C.: Specific interaction of ions. Trans. Faraday Soc. 51, 747–761 (1935)
Bromley, L.A.: Thermodynamic properties of strong electrolytes in aqueous solutions. AIChE J. 19, 313–320 (1973)
Pitzer, K.S.: Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Pitzer, K.S.: Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300–2308 (1973)
Cruz, J.L., Renon, H.: A new thermodynamic representation of binary electrolyte solutions nonideality in the whole range of concentrations. AIChE J. 24, 817–830 (1978)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Chen, C.C., Britt, H.I., Boston, J.F., Evans, L.B.: Local composition model for excess Gibbs energy of electrolyte systems. Part I: Single solvent, single completely dissociated electrolyte systems. AIChE J. 28, 588–596 (1982)
Chen, C.C., Evans, L.B.: A local composition model for the excess Gibbs energy of aqueous electrolyte systems. AIChE J. 32, 1655–1664 (1986)
Haghtalab, A., Vera, J.H.: A nonrandom factor model for the excess Gibbs energy of electrolyte solutions. AIChE J. 34, 803–813 (1988)
Thomsen, K., Rasmussen, P., Gani, R.: Correlation and prediction of thermal properties and phase behavior for a class of aqueous electrolyte systems. Chem. Eng. Sci. 51, 3675–3683 (1996)
Jaretun, A., Aly, G.: New local composition model for electrolyte solutions: single solvent, single electrolyte systems. Fluid Phase Equilib. 163, 175–193 (1999)
Jaretun, A., Aly, G.: New local composition model for electrolyte solutions: multicomponent systems. Fluid Phase Equilib. 175, 213–228 (2000)
Zhao, E., Yu, M., Sauvé, R.E., Khoshkbarchi, M.K.: Extension of the Wilson model to electrolyte solutions. Fluid Phase Equilib. 173, 161175 (2000)
Sadeghi, R.: New local composition model for electrolyte solutions. Fluid Phase Equilib. 231, 53–60 (2005)
Messnaoui, B., Ouiazzane, S., Bouhaouss, A., Bounahmidi, T.: A modified electrolyte–UNIQUAC model for computing the activity coefficient and phase diagrams of electrolytes systems. CALPHAD 32, 566–576 (2008)
Haghtalab, A., Peyvandi, K.: Electrolyte-UNIQUAC-NRF model for the correlation of the mean activity coefficient of electrolyte solutions. Fluid Phase Equilib. 281, 163–171 (2009)
Haghtalab, A., Peyvandi, K.: Generalized electrolyte-UNIQUAC-NRF model for calculation of solubility and vapor pressure of multicomponent electrolytes solutions. J. Mol. Liq. 165, 101–112 (2012)
Haghtalab, A., Asadollahi, M.A.: An excess Gibbs energy model to study the phase behavior of aqueous two-phase systems of polyethylene glycol + d-extran. Fluid Phase Equilib. 171, 77–90 (2000)
Hildebrand, J.H., Scott, R.L.: The Solubility of Non-electrolytes, 3rd edn. Reinhold Publishing Corporation, New York (1950)
Guggenheim, E.A.: Mixtures. Oxford University Press, Oxford (1952)
Song, Y., Chen, C.C.: Symmetric electrolyte nonrandom two-liquid activity coefficient model. Ind. Eng. Chem. Res. 48, 7788–7797 (2009)
Christensen, C., Sander, B., Fredenslund, A.A., Rasmussen, P.: Towards the extension of UNIFAC to mixtures with electrolytes. Fluid Phase Equilib. 13, 297–309 (1983)
Mazloumi, S.H.: Representation of activity and osmotic coefficients of electrolyte solutions using a non-electrolyte Wilson-NRF model with ion-specific parameters. Fluid Phase Equilib. 388, 31–36 (2015)
Mazloumi, S.H.: An ion-based nonelectrolyte NRTL-NRF for aqueous electrolyte solutions. CALPHAD 51, 299–305 (2015)
Mazloumi, S.H.: On the application of the nonelectrolyte-UNIQUAC-NRF model for strong aqueous electrolyte solutions. Fluid Phase Equilib. 417, 70–76 (2016)
Ahmadi, H., Peyvandi, K.: Electrolyte-UNIQUAC-NRF model based on ion specific parameters for the correlation of mean activity coefficients of electrolyte solutions. J. Solution Chem. 46, 1202–1219 (2017)
Kuramochi, H., Osako, M., Kida, A., Nishimura, K., Kawamoto, K., Asakuma, Y., Fukui, K., Maeda, K.: Determination of ion-specific NRTL parameters for predicting phase equilibria in aqueous multi electrolyte solutions. Ind. Eng. Chem. Res. 44, 3289–3297 (2005)
Thomsen, K., Rasmussen, P.: Modeling of vapor–liquid–solid equilibrium in gas– aqueous electrolyte systems. Chem. Eng. Sci. 54, 1787–1802 (1999)
Hamer, W.J., Wu, Y.C.: Osmotic coefficients and mean activity coefficients of uni-univalent electrolytes in water at 25°C. J. Phys. Chem. Ref. Data 1, 1047–1099 (1972)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, 2nd edn. Dover Publications Inc, New York (2002)
Goldberg, R.N.: Evaluated activity and osmotic coefficients for aqueous solutions: thirty-six uni-bivalent electrolytes. J. Phys. Chem. Ref. Data 10, 671–764 (1981)
Goldberg, R.N., Nuttall, R.L.: Evaluated activity and osmotic coefficients for aqueous solutions: the alkaline earth metal halides. J. Phys. Chem. Ref. Data 7, 263–310 (1978)
Lagarias, J.C., Reeds, J.A., Wright, M.H., Wright, P.E.: Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM J. Optim. 9, 112–147 (1998)
Clarke, E.C.W., Glew, D.N.: Evaluation of thermodynamic functions from equilibrium constants. Trans. Faraday Soc. 62, 539–547 (1996)
Zaytsev, I.D., Aseyev, G.G.: Properties of Aqueous Solutions of Electrolytes. CRC Press, Florida (1992)
Pitzer, K.S., Pelper, J.C., Busey, R.H.: Thermodynamic properties of aqueous sodium chloride solutions. J. Phys. Chem. Ref. Data 13, 1–102 (1984)
Archer, D.G.: Thermodynamic properties of the NaBr + H2O system. Phys. Chem. Ref. Data 20, 509–555 (1991)
Amado, G.E., Blanco, L.H.: Osmotic and activity coefficients of aqueous solutions of KCl at temperatures of 283.15, 288.15, 293.15 and 298.15 K, A new isopiestic apparatus. Fluid Phase Equilib. 226, 261–265 (2004)
Pabalan, R.T., Pitzer, K.S.: Heat capacity and other thermodynamic properties of Na2SO4(aq) in hydrothermal solutions and the solubilities of sodium sulfate minerals in the system Na-Cl–SO4–OH–H2O to 300 °C. Geochim. Cosmochim. Acta 52, 2393–2404 (1988)
Baabor, S., Gilchrist, M.A., Delgado, E.J.: Isopiestic study of (calcium chloride + water) and (calcium chloride + magnesium chloride + water) at T = 313.15 K. J. Chem. Thermodyn. 33, 405–411 (2001)
Prausnitz, J.M., Lichtenthaler, R.N., de Azevedo, E.G.: Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd edn. Prentice Hall PTR, New Jersey (1999)
Wagman, D.D., Evans, W.H., Parker, V.B., Schumm, R.H., Bailey, S.M., Halow, I., Churney, K.L., Nuttal, R.L.: The NBS tables of chemical thermodynamic properties. Selected values for inorganic and C1 and C2 organic substances in SI units. J. Phys. Chem. Ref. Data 11(Suppl. 2), 1–392 (1982)
Linke, W.F., Seidell, A.: Solubilities of Inorganic and Metal-Organic Compounds, 4th edn. American Chemical Society, Washington (1965)
Potter II, R.W., Clynne, M.A.: Solubility of NaCl and KCI in aqueous HCI from 20 to 85 °C. J. Chem. Eng. Data 25, 50–51 (1980)
El Guendouzi, M., Benbiyi, A., Dinane, A., Azougen, R.: The thermodynamic study of the system LiCl–KCl–H2O at the temperature 298.15 K. CALPHAD 27, 213–219 (2003)
Marouani, M., El Guendouzi, M.: Determination of water activities, osmotic and activity coefficients of the system NH4NO3–LiNO3–H2O at the temperature 298.15 K. CALPHAD 28, 321–327 (2004)
El Guendouzi, M., Azougen, R., Dinane, A., Benbiyi, A.: Hygrometric determination of the water activities of the aqueous solutions of the mixed electrolyte NaCl–CaCl2–H2O at 25 °C. J. Solution Chem. 33, 941–955 (2004)
Patil, K.R., Tripathi, A.D., Pathak, G., Katti, S.S.: Thermodynamic properties of aqueous electrolyte solutions. 1. Vapor pressure of aqueous solutions of lithium chloride, lithium bromide, and lithium iodide. J. Chem. Eng. Data 35, 166–168 (1990)
Nasirzadeh, K., Neueder, R., Kunz, W.: Vapor pressures and osmotic coefficients of aqueous LiOH solutions at temperatures ranging from 298.15 to 363.15 K. Ind. Eng. Chem. Res. 44, 3807–3814 (2005)
Robinson, R.A., Platford, R.F., Childs, C.W.: Thermodynamics of aqueous mixtures of sodium chloride, potassium chloride, sodium sulfate, and potassium sulfate at 25 °C. J. Solution Chem. 1(167–172), 65989740 (1972)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Razavi, S.M., Haghtalab, A. & Khanchi, A.R. An Electrolyte Non-random-UNIQUAC Model for Thermodynamic Modeling of Binary and Multicomponent Aqueous Electrolyte Systems. J Solution Chem 48, 624–657 (2019). https://doi.org/10.1007/s10953-019-00876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00876-0