Abstract
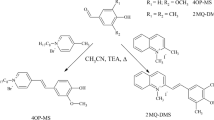
Solvatochromic studies on two new merocyanines, prepared from 5-(5-bromo-6-hydroxynaphthyl-2)-7,7-dimethyl-7H-indolo[1,2-a]quinolinium perchlorate and 5-(5-nitro-6-hydroxynaphthyl-2)-7,7-dimethyl-7H-indolo[1,2-a]quinolinium perchlorate, were performed in 21 solvents using UV–vis spectroscopy. The VBHB model (J.A. Soroka and K.B. Soroka in J. Phys. Org. Chem. 4:592–604, 1991) was applied to describe their solvatochromic properties.
The results indicate that both dyes exhibit variable solvatochromism, i.e. it is positive in nonpolar solvents and negative in polar aprotic and protic solvents. The solvatochromic shifts of both merocyanines are approximately 8000 cm−1(for toluene to water). The studies revealed that these two merocyanines exist in solutions as nonpolar (quinoid, i.e. vinylogous amide, V) or polar (zwitterionic, i.e. betaine, B) forms in their ground state, respectively. The dye with a nitro substituent can also form a hydrogen-bonded betaine (HB). This dye is found to be the first compound in the whole group of 7H-indolo[1,2-a]quinolinium merocyanines that does not comply with the VBHB model assumptions.







Similar content being viewed by others
References
Reichardt, Ch.: Solvents and Solvent Effects in Organic Chemistry, 3th edn. VCH, Weinheim (2003)
Soroka, J.A., Soroka, K.B.: Solvatochromism of dyes. Part I. Solvatochromism of merocyanines. Derivatives of the 7H-indolo[1,2-a]quinolinium system. A new model of solvatochromism. J. Phys. Org. Chem. 4, 592–604 (1991)
Wróblewska, E.K., Soroka, J.A., Soroka, K.B., Gąsiorowska, M.: Solvatochromism of dyes. Part IV—Energetic characteristic of merocyanine derivatives of 1-phenyl-2-[2-(3-X-4-hydroxy-5-R-phenyl)ethenyl]-3,3-dimethyl-3H-indolium cation. J. Phys. Org. Chem. 18, 347–352 (2005)
Dimroth, K., Reichardt, Ch., Siepmann, T., Bohlmann, F.: Über Pyridinium-N-phenol-betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln. Liebigs Ann. Chem. 661, 1–37 (1963)
Dimroth, K., Reichardt, Ch.: Über Pyridinium-N−phenol-betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln. V. Erweiterung der Lösungsmittelpolaritätsskala durch Verwendung Alkyl-substituierter Pyridinium-N-phenol-betaine. Justus Liebigs Ann. Chem. 727, 93–105 (1969)
Sawicka, M.J., Soroka, J.A., Wróblewska, E.K., Zawadzka, I.K.: Synthesis and UV–vis study of a new strongly solvatochromic merocyanine-like dye with modified donor part. Pol. J. Chem. 80, 1337–1351 (2006)
Sawicka, M.J., Soroka, J.A., Gąsiorowska, M.: A new method for the preparation of solvatochromic 5-(5-X-6-hydroxynaphthyl-2)-7H-indolo[1,2-a]quinolinium merocyanines Pol. J. Chem. Technol. 12(1), 17–22 (2010)
Chandra, A.K., Uchimaru, T.: The O–H bond dissociation energies of substituted phenols and proton affinities of substituted phenoxide ions: a DFT study. Int. J. Mol. Sci. 3, 407–422 (2002)
Pimentel, G., McClellan, A.: The Hydrogen Bond. Freeman, San Francisco (1960)
Jiang, L., Lai, L.: CH⋯O hydrogen bonds at protein–protein interfaces. J. Biol. Chem. 277(40), 37732–37740 (2002)
Domagała, M., Grabowski, S.J.: Hydrocarbons as proton donors in C–H⋯N and C–H⋯S hydrogen bonds. Chem. Phys. 367, 1–6 (2010)
Zierkiewicz, W.: Modelling of interactions between volatile anesthetics (halothane, enflurane) and aromatic compounds, ab initio study. Chem. Phys. 373, 243–250 (2010)
Wojtulewski, S., Grabowski, S.J.: Different donors and acceptors for intramolecular hydrogen bonds. Chem. Phys. Lett. 378, 388–394 (2003)
Gore, M.G.: Spectrophotometry & Spectrofluorimetry. Practical Approach. Oxford University Press Inc., New York (2000)
Wróblewska, E.K., Soroka, J.A., Soroka, K.B., Gąsiorowska, M., Sawicka, M.J.: Calibration surfaces in analysis of ternary mixtures. Part 2. Degree of analytical utility of solvatochromic indicator. Chem. Anal. (Warsaw) 49, 729–738 (2004)
Lang, L.: Absorption spectra in the ultraviolet and visible region. XIV, No 2480, Académiai Kiadó, Budapest (1970)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawicka, M.J., Soroka, J.A., Gąsiorowska, M. et al. The Spectroscopic Behavior of Two New 5-(5-R-6-Hydroxynaphthyl-2)-7,7-dimethyl-7H-indolo[1,2-a]quinolinium Merocyanines in Various Solvents. J Solution Chem 41, 25–35 (2012). https://doi.org/10.1007/s10953-011-9778-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9778-z