Abstract
Rubidium (Rb) has been shown to impact biological activity. This work synthesized Rb-doped mesoporous bioactive glass nanoparticles (MBGNs) based on the composition 70SiO2–30CaO mol% with a sol-gel method. Rb2O was substituted for CaO in concentrations of 5 and 10 mol%. The influence of Rb incorporation on the hydroxycarbonate apatite (HCA) formation, cytotoxicity, and antibacterial capacity of particles was evaluated. XRD analysis confirmed the amorphous structure of the particles. In vitro, biomineralization studies showed HCA on the surface of MBGN and Rb-doped MBGN pellets after 7 days of soaking in simulated body fluid (SBF). An inhibition zone of Escherichia coli (E.coli) and Staphylococcus aureus (S. aureus) around Rb-doped MBGN pellets was detected, while MBGN pellets did not show any inhibition zone. Additionally, MC3T3-E1 pre-osteoblastic cells demonstrated cytocompatibility when exposed to Rb-MBG suspensions at different concentrations of up to 250 µg/ml. Based on their overall properties, Rb-containing MBGNs are proposed for biomedical applications, such as filler nanoparticles in composite bone scaffolds.
Similar content being viewed by others
1 Introduction
Mesoporous bioactive glass nanoparticles (MBGNs) have been widely researched and considered as bioactive fillers and drug carrier regulators for bone regeneration because of their outstanding biocompatibility, high bioactivity, and nanostructured morphology [1]. Owing to their excellent textural properties, MBGNs significantly enhance HCA formation in contact with biological fluids and thus promote apatite mineralization when compared to dense bioactive glass particles with the same composition [2,3,4]. Furthermore, the specific surface area and pore volume of MBGNs provide advantages for loading and delivering different drugs (e.g., antibiotic and anti-cancer agents) and bioactive molecules (e.g., growth factors and genes) [5, 6].
The sol-gel method is a versatile process to synthesize highly ordered MBGNs due to its simplicity, low-temperature processing, and high purity of the synthesized materials [7]. The textural properties, particle size, and density of MBGNs are influenced by the combination of the sol-gel process and template used [1, 8]. For designing the textural characteristics of MBGNs and influencing their bioactivity, the templates require careful selection [1, 5, 8, 9]. In 2004, sol-gel-derived mesoporous bioactive glasses were prepared initially by Yan et al. [3]. Since that study, many material scientists have widely investigated mesoporous bioactive glasses as drug carriers and for other biomaterial applications [6, 10, 11].
Several therapeutic ions, such as copper (Cu), zinc (Zn), boron (B), silver (Ag), and strontium (Sr) [12,13,14,15,16], have been successfully incorporated in sol-gel derived bioactive glasses to develop multifunctional properties. For example, Ag+ and Cu2+ exhibit effectiveness against a wide range of bacteria. However, high doses of Ag+ or Cu2+ ions can negatively affect osteoblasts by inhibiting their proliferation and differentiation [17, 18].
Rubidium (Rb) is an alkali element found in some human organs [19], and Rb might be beneficial for its neurophysiological function and immune response [20,21,22]. In the field of biomaterials, Rb is a biologically active ion that improves physicochemical and biological properties [23,24,25]. Ouyang et al. [26] synthesized monodispersed Rb-doped bioactive glass nanospheres (BGN-Rb). Their study showed that after 3 days of immersion in SBF, BGN-5Rb exhibited significantly improved apatite-forming ability, inducing apatite crystal growth on the BG surfaces [26]. For further study, Tan et al. [27] successfully prepared Rb-containing bioactive glass-ceramic in the CaO-SiO2-Na2O-B2O3-MgO-ZnO-P2O5 system. Their results showed that the incorporation of Rb into glass-ceramics improved the mineralization, bending strength, cell proliferation, and adhesion, as well as the osteogenic differentiation ability of human bone marrow mesenchymal stem cells (hBMSCs).
Previous studies [26, 27] have reported the potential of incorporating Rb into dense bioactive glasses and glass-ceramics for bone regeneration application. However, the synthesis of Rb-substituted bioactive glasses with an ordered mesoporous structure has not been thoroughly investigated regarding its physicochemical and biological properties. Therefore, this research aims to address this gap by synthesizing a new series of MBGNs with a composition of 70SiO2 – 30CaO (in mol%), containing Rb2O at concentrations of 5 and 10 mol%, using the sol-gel method. The study focused on examining the effects of Rb addition on morphological characteristics, HCA formation, cytotoxicity of pre-osteoblastic cells (MC3T3-E1), and antibacterial properties against E.coli and S. aureus. We hypothesize that Rb-doped MBGNs are promising for biomedical applications, such as biodegradable drug carriers and filler nanoparticles, particularly for hydrogels, for guided bone regeneration applications.
2 Materials and methods
2.1 Precursor
Cetyltrimethylammonium bromide (CTAB; ≥97%) and ethyl acetate (EA; ≥ 99.8%) were purchased from Merck, Germany. Ammonium hydroxide (28%), ethanol (96%), and calcium nitrate tetrahydrate (CNT; 99.5%) were obtained from VWR, France, while tetraethyl orthosilicate (TEOS; 99%), as well as rubidium chloride (99.8%), were supplied by Sigma-Aldrich, Germany.
2.2 Rb-doped Mesoporous bioactive glass nanoparticle synthesis
Rb-doped MBGNs based on the composition 70SiO2 – 30CaO (in mol%) were synthesized using a microemulsion-assisted sol-gel method as reported previously [13]. Calcium oxide (CaO) was substituted in varying amounts with rubidium oxide (RbO2), as shown in Table 1. In this study, calcium nitrate tetrahydrate was selected as a calcium source, and rubidium chloride was chosen as a rubidium source for synthesizing sol-gel-derived bioactive glasses. This choice was made based on the observation that the bioactive glass, produced using calcium nitrate tetrahydrate and rubidium chloride, exhibited an amorphous nature after sintering in the temperature range of 400–700 °C [26, 28, 29].
The synthesis of Rb-MBGNs was initiated by dissolving 5.6 g of CTAB in 264 ml of deionized water (DI water) and stirring until the solution turned colorless. The following steps were carried out at room temperature under continuous stirring. The clear solution was mixed with 80 ml of ethyl acetate for 30 min. After adjusting with 56 ml of 1 M ammonia solution, the solution was stirred for 15 min. Later, 28.8 ml of TEOS was gradually added and stirred for 30 min. Next, the proper amount of calcium nitrate tetrahydrate and RbCl were sequentially dissolved into the mixture, stirring every 30 min. The solution was stirred for 4 h; white precipitates were visible. The mixture was then centrifuged. The supernatant was poured off, and white precipitates were washed three times with DI water and once with ethanol. The washed particles were dried at 60 °C for at least 24 h. Finally, the dried particles were calcined at 700 °C for 3 h with a heating rate of 2 °C/min to achieve the Rb-containing MBGNs. MBGNs without RbO2 were prepared following the same process; however, the addition of rubidium chloride was excluded.
2.3 Mesoporous bioactive glass nanoparticle characterizations
X-ray diffraction (XRD) analysis (Miniflex 600 h, Rigaku, Japan) was conducted in the 2θ range from 5° to 60° with a step size of 0.02°. The morphology of the particles was characterized by scanning electron microscopy (SEM; Zeiss Auriga 4750) at an accelerating voltage of 1.5 kV and a working distance of ∼4 mm. FTIR spectra of particles were obtained using a FTIR Spectrometer (IRAffinity-1 S, SHIMADZU, Germany) in the range of 400–4000 cm− 1.
Chemical compositions of bioactive glass samples were determined using X-ray Fluorescence (XRF; S8 Tiger, Bruker AXS, Germany) wavelength-dispersive spectrometer equipped with a rhodium X-ray tube. The samples were scanned in the X-ray tube under vacuum conditions, operating at a power of 1 kW, with a voltage of 50 kV and a current of 50 mA. Before the XRF measurement, the sample was prepared into a pressed pellet with diameter of 40 mm. Image J analysis software (NIH, Bethesda, MD, USA) was employed for size analysis, with mean diameter size values calculated based on counting at least 400 particles for each composition with varying amounts of doped Rb (n = 3 per sample). Additionally, the textural properties of the particles were determined using the nitrogen sorption method at 77 K, conducted with a Quadraorb™ SI gas adsorption analyzer. This thorough characterization provides valuable insights into the structural, morphological, compositional, and textural properties of the studied MBGNs.
2.4 HCA formation in simulated body fluid (SBF)
In vitro HCA formation on the surface of MBGNs is an indicator of the material bioactivity, and it indicates the potential of the materials to bond with bone tissue. SBF solution was prepared by the protocol described by Kokubo et al. [30]. Briefly, 0Rb, 5Rb and 10Rb pellets of 1.5 cm diameter (200 mg) were prepared by uniaxial pressing at 1 ton for 1 min. Then, the pellets were immersed in 150 ml of SBF and were shaken at 90 rpm for 1,3,7, 14 and 30 days at 37 °C. The pH value of SBF was also measured at 1, 3, 7, and 14 days during the soaking period. Supernatants with 30 ml were collected at intervals of 7, 14, and 30 days through centrifugation and followed by filtration. The pH of the collected samples was adjusted to 2 using 1 M HCl. Subsequently, the concentrations of released Si, Ca, and Rb of samples were determined by ICP-OES (Agilent 5100 SVDV, Sta. Clara, USA).
After 7 days, SBF was gently rinsed off and washed with DI water twice. At this point, samples were dried in an oven at 60 °C for 24 h. FTIR, XRD, SEM, and EDS analyses were used to assess HCA formation on the surface of samples.
2.5 In vitro cytotoxicity
The in vitro cytotoxicity of the MBGNs was investigated by the WST-8 cell proliferation assay kit (Sigma Aldrich, Germany) using the direct contact method according to the ISO-10993-5 standard [31]. Firstly, MC3T3-E1 pre-osteoblastic cells (Sigma Aldrich, Germany) were cultured in Minimum essential medium (α-MEM, Gibco, UK) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, Germany), 1% penicillin /streptomycin (PS, Gibco, Germany), and 1%L-glutamine (Gibco, Germany) and incubated at 37 ºC in 5% CO2. Then, MC3T3-E1 pre-osteoblastic cells were seeded in 24-well plates at a 105 cells/well density and incubated for 24 h. In parallel, samples were sterilized in a furnace (Muffle furnace L3/11, Nabertherm) for 2 h at 160 °C. After 24 h incubation, each well’s cell culture medium was exchanged with a cell culture medium containing sterile particles (1 ml) at concentrations 1000, 500, 250, 100, and 50 µg/ml. Then, the cells were incubated for another 48 h. After 48 h of incubation, the cell viability was analyzed by WST-8 assay (1% (v/v), and the absorbance was measured at 450 nm by a microplate reader (PHOmo, Anthos Microsystem GmbH, Germany). The cell viability was calculated according to the following equation (Eq. 1):
MC3T3-E1 pre-osteoblastic cells without MBGNs were used as a reference and labeled as “TCP”. The blank was the WST-8 solution. The results were normalized concerning the reference sample, and experiments were performed in four replications. Following a 48 h incubation at 37 ºC, the pH values of the medium with particles were recorded.
2.6 Antibacterial assay
The antibacterial activity of the synthesized MBGNs was tested with S. aureus (ATCC 25923, gram-positive) and E. coli (ATCC 25922, gram-negative) bacteria, and the inhibition zone of the pellets was assessed by the agar diffusion method according to Standard ' SNV 195920-1992’. Briefly, MBGN and Rb-MBGN powders (200 mg) were placed in a metallic mold with an inner diameter of 1.5 cm and uniaxially pressed under 1 ton for 1 min to prepare pellets. Further, the pellets were sterilized in a furnace for 2 h at 160 °C. Meanwhile, both strains of bacteria were inoculated in Luria/Miller medium for 24 h at 37 °C. Then, the optical density (OD) of the bacteria population was calibrated (600 nm, Thermo Scientific™ GENESYS 30™, Germany) to reach the value of 0.015, according to bacterial turbidity measurement cultures [32]. Afterward, a volume of 20 µl inoculum of S. aureus and E. coli was spread evenly over the agar plate’s entire surface separately. Then, the pellets were placed on the plate and incubated for 24 h at 37 °C. After 24 h of incubation, the inhibition zone of the pellets was measured by Image J analysis software. Each pellet was replicated three times.
2.7 Statistical analysis
Statistical analyses were performed using the paired comparison plot (Tukey’s test) in software Origin 2022. All experiments were performed at least four times. Statistical significance is shown in * p < 0.05, ** p < 0.01, and *** p < 0.001. Experimental results were reported as mean ± standard deviation.
3 Results and discussion
3.1 Characterization of Rb-MBG nanoparticles
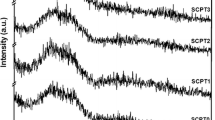
The structural evaluation of MBGNs with rubidium doping is presented in Fig. 1. In Fig. 1(a) shows XRD patterns of MBGNs with varying concentrations of Rb doping. All the XRD patterns exhibit a broad band in the range of 20° − 34° (2θ) [13], indicating an amorphous phase of the MBGNs both with and without Rb2O doping. Additionally, Fig. 1(b) illustrates the FTIR spectra of the 0Rb, 5Rb and 10Rb samples after calcination at 700 °C. The FTIR spectra of the samples after calcination show the most predominant bands corresponding to the vibrations of Si-O-Si network at around 450 cm− 1, 800 cm− 1, and 1000–1200 cm− 1 [8, 13, 26]. Some differences in the FTIR spectra of various compositions are clearly apparent. The substitution of Rb2O for CaO in the glass composition can result in changes in the vibrational frequencies as well as the intensity of the bands associated to the Si-O-Si network vibrations, indicating changes in the network structure of the MBGNs. These modifications are influenced by the interaction between Rb+, Ca2+ and the silicate network structure. The glass network of the studied compositions consists of SiO4 tetrahedra with Si-O-Si bridging bonds, where the network modifier Rb+ and Ca2+ ions are located in the interstices of the network. Higher Rb+ addition to MGBNs is anticipated to break more Si-O-Si bonds, resulting in a decrease in the overall intensity of the bands associated with the SiO4 network. The shift in the position of the Si-O-Si stretching band is also seen in the FTIR spectra of different Rb+ concentrations. This shift indicates changes in the bond length and angles in the glass network owing to the larger size of the Rb+ ions being substituted for the smaller size of the Ca2+ ions. Furthermore, XRF analysis confirmed the successful doping of Rb into MBGNs at various concentrations, as summarized in Table 1. Notably, the actual Rb content was lower than the one indicated by the nominal composition. This difference can be explained by the formation of bioactive glass nanoparticles using the sol-gel process. During our synthesis of MBGNs, Ca and Rb precursors were introduced after the initial formation of silicate particles, driven by the limitation of the adsorption capability of both ions on the silicate particle surface. Moreover, the loosely adsorbed ions were subsequently eliminated from the nanoparticles during the washing step, a crucial process essential for ensuring the uniform dispersity of the particles [26, 33, 34].
This study employed a microemulsion-based sol-gel technique to produce ordered porous structures of Rb-doped MBGNs. SEM technique was performed to investigate the morphology and structure of all particles. Figure 2 shows the SEM images of MBGNs and Rb-doped MBGNs, revealing that the nanoparticles exhibited nearly spherical or ellipsoidal shape, measuring approximately 50 to 200 nm in diameter, which is similar to previous studies [9, 35]. Both morphologies could be caused by the fusion of microemulsion droplets from ethyl acetate during the synthesis process [9, 36, 37]. CTAB is used as a pore template for the synthesis of MBG, and thus, the pores on the surface of MBG could be due to CTAB burnout during the calcination process [1].
The nitrogen adsorption analysis confirmed the mesoporous structure of particles, as indicated by the nitrogen adsorption/desorption isotherms of 0Rb, 5Rb, and 10Rb shown in Fig. 3. These isotherms were identified as type IV with a H1 hysteresis loop, characteristic of mesoporous materials exhibiting cylindrical-shaped pores [8, 11, 38]. Textural properties, including the specific surface area and pore size, were determined by Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods. The average pore diameter for all samples was 5 nm. This value is consistent with literature values when CTAB is used as a pore templating agent [39]. The specific surface area increased slightly with Rb incorporation: 243, 268, and 276 m2/g for 0Rb, 5Rb and 10Rb, respectively. Consequently, the relatively high specific surface areas of 5Rb and 10Rb could enhance the dissolution rate and ion exchange processes serving as nucleation sites for HCA formation, promoting bioactivity, compared with 0Rb.
3.2 HCA formation in SBF
To investigate the formation of HCA, pellets made from different types of MBGNs (0Rb, 5Rb, and 10Rb) were submerged in SBF solution for 7 days. The confirmation of the HCA layer was achieved through characterization by FTIR, XRD and SEM.
XRD patterns in Fig. 1(c) revealed the HCA formation of 0Rb, 5Rb, and 10Rb groups, by presenting crystalline peaks at 2θ values 26°, 32° and 45.5°. These peaks identified hydroxyapatite as a crystalline phase (JCPDS 09-0432) [40]. The confirmation was further supported by FTIR analysis, as shown in Fig. 1(d), where spectra for 0Rb, 5Rb, and 10Rb groups showed double P-O bands at 560 and 600 cm-1 [26, 41] and a new PO43- band located at 960 cm-1 [42]. Additionally, a band at 874 cm-1 in the FTIR spectra [43] was assigned to the carbonate group, indicating carbonate substitution in some parts of the apatite crystalline structure. However, the XRD technique does not allow the distinction between hydroxyapatite and hydroxycarbonate apatite.
SEM images in Fig. 4 reveal the morphology of HCA on the pellet surface. After soaking 7 days in SBF, the entire surface of the 0Rb, 5Rb, and 10Rb pellets was completely covered with HCA precipitation, showing a typical needle-like structure. Due to the nano sizes of mesoporous bioactive glasses, HCA crystals on the surface of pellets could not form a typical cauliflower-like morphology which is usually found on other BG-based materials [13, 17]. This behaviour is consistent with results of previous studies [13, 44]. Interestingly, we observed numerous clumps of needle-like nanorod structures (HCA) coming together in the 5Rb and 10Rb pellets, indicating that these samples have a highly crystalline structure. Additionally, the Ca/P ratio of 0Rb, 5Rb, and 10Rb was evaluated through EDS. After 7-days incubation in SBF, the Ca/P ratio for samples 0Rb, 5Rb and 10Rb was close to 1.67. This finding indicates the presence of HCA, as the Ca/P ratio closely aligns with the theoretical value of stoichiometric hydroxycarbonate apatite.
In the bioactivity assay, pH values were measured in the SBF solution, as illustrated in Fig. 5(a). Across all samples (0Rb, 5Rb, and 10Rb), a trend of pH increase was observed over the incubation period. This rise in pH can be attributed to the exchange of cations from the bioactive glass with protons from the SBF solution, leading to an increase of the concentration of hydroxyl ions and consequently elevating the overall pH of the solution [45].
The ionic profiles of Si, Ca and Rb are shown in Fig. 5(b and c). Si species were released into SBF due to the degraded silica network of the MBGNs. For 0Rb and 10Rb, the Si concentration ranged between 55 and 60 mg/L throughout the experiment. It is noteworthy that the Si concentration in our study fell within the range 0.1–100 mg/L, which can have a stimulating effect on osteoblast cells [46]. Concerning the release of Ca in SBF, a reduction in the release rate was observed for 0Rb and 10Rb samples after 3 days, confirming the deposition of hydroxycarbonate apatite. Finally, the Rb concentration from 10Rb did not exceed 20 mg/L after 30 days of testing.
Overall, the results collectively highlight the successful generation of the HCA layer on pellets made from Rb-doped MBGNs immersed in SBF for 7 days and emphasize the potential bioactivity of Rb-doped MBGNs.
3.3 Cytotoxicity
The cytotoxicity of 0Rb, 5Rb, and 10Rb samples was investigated by direct contact assay using the WST-8 cell-culture kit with MC3T3-E1 pre-osteoblastic cells. The MC3T3-E1 pre-osteoblastic cells were treated with various concentrations of samples (direct method) for 48 h, and TCP served as the control. Following the ISO 10,993 standard, the cell viability of a biocompatible material should be equal to or higher than 70% of the control [46]. As seen in Fig. 6(a), at concentrations 500 and 1000 µg/ml of all samples, the MC3T3-E1 pre-osteoblastic cells showed reduced cell viability (less than 70%). The results indicate that both high concentrations of nanoparticles were not biocompatible with the MC3T3-E1 cells. However, 0Rb, 5Rb, and 10Rb were not cytotoxic to the MC3T3-E1 cells at concentrations between 50 and 250 µg/ml (cell viability of more than 70%). At these concentrations, 5Rb and 10Rb showed higher cell viability than 0Rb nanoparticles, which slightly increased with an increasing concentration of Rb. As shown in Fig. 6(b), the pH of the α-MEM medium after immersion of the samples for 48 h was in the range 7.40–7.48, which is in a recommended pH range of 7.40–7.60 for optimal osteoblast activity [47, 48]. Thus it was confirmed that the pH values of the suspensions did not induce negative effects on cells. Indeed the Si, Ca, and Rb ions released from MBGNs during their dissolution process can mainly impact cell viability. Previous studies demonstrated that the release of Rb ions positively affects the growth and proliferation of human osteosarcoma cells (MG-63 cells) in a dose-dependent manner [25]. The authors’ next study confirmed by MTT assay that human bone marrow stromal cell viability increased by incorporating Rb (10% Rb) into the glass-ceramic compared to the glass-ceramic without Rb doping [27]. In another related study, Naruphontjirakul et al. [49] evaluated the effect of Sr-containing BG nanoparticles (direct method) on the viability of MC3T3-E1 cells. The results showed no toxic effect with Sr-nanoparticle concentrations between 0.01 and 250 µg/ml. However, a significant reduction in cell viability was observed at nanoparticle concentrations greater than 500 µg/ml. Their finding aligns with our results, showing that BG nanoparticles up to 250 µg/ml concentrations were biocompatible with MC3T3-E1 pre-osteoblastic cells. A further investigation to consider the possible internalization of the nanoparticles by the cells should be carried out in the future.
(a) Viability of MC3T3-E1 pre-osteoblastic cells treated with the cell culture medium containing MBGNs (direct) at different concentrations for 48 h of incubation, (b) pH value of α-MEM medium after incubation of MBGNs for 48 h at 37°C. The asterisks indicate a significant difference (* p <= 0.05, ** p <= 0.01, and *** p <= 0.001). (n=6 per group)
3.4 Antibacterial properties
The antibacterial activity of pellets made from MBGNs with varying Rb content was evaluated by agar diffusion technique against E. coli and S. aureus bacteria. These typical bacteria can cause bacterial infections when the biomaterial is in contact with human tissue. Bacterial infections are serious problems in patients after surgery [6]. For example, S.aureus can form biofilms on the surface of bone implants, a common cause of failure of bone healing [50]. In this study, the efficiency of antibacterial properties is expressed in terms of the average diameter of the inhibition zone of bacteria growth surrounding the pellets. According to standard ‘SNV 195920-1992’, an inhibition zone greater than 1 mm is considered to indicate antibacterial potential [32]. As shown in Fig. 7, the 0Rb pellet did not produce a bacterial inhibition zone for both bacterial strains. However, high bacterial inhibition zones around the 5Rb and 10Rb pellets were observed. The results showed a slight increase in the growth inhibition zones against E. coli with increasing concentration of Rb, ranging from 5 mol% to 10 mol%. Thus, the release of Rb ions imparts antibacterial properties against E. coli. and S. aureus. In a previous study, Liu et al. [25] demonstrated the antibacterial activity of Rb-containing nano-hydroxyapatite (Rb-nHAp) against E.coli (ATCC8739) and S. aureus (ATCC6538). Their results showed that the number of E.coli and S. aureus bacterial cells on Rb-nHAp was significantly lower than those of pure HAp and the control group, which agrees with our experimental results. The authors suggested that the antibacterial capacity results might be linked to the Rb ion release from the materials. The Rb ion release could cause leakage from E.coli and S.aureus bacteria, leading to bacterial cell death. Generally, the potassium (K) ion channel induces bacteria growth and metabolism as well as controls the formation of bacterial biofilm [25]. Chen et al. [23] reported that Rb ions have a certain antibacterial capacity linked to the inhibitory potassium channel of bacteria. Due to electrostatic attraction between Rb (positive charge) and the bacteria (negative charge), Rb ions could be absorbed on the bacterial surfaces.
Moreover, Rb ions could substitute K ions of the bacteria membranes and could block K-channels, leading to cell damage and apoptosis [23]. In cases of BGs, they increase the pH in physiological fluid, resulting in the inhibition of bacteria growth, contributing to the antibacterial properties of BGs [51]. These findings support the strategy of incorporating at least 5 mol% rubidium into MBGNs to enhance the antibacterial activity of MBGNs, thereby expanding their application potential.
4 Conclusions
This study successfully synthesized Rb-doped MBGNs via a microemulsion-assisted sol-gel process, utilizing cetyltrimethylammonium bromide as a template agent. The nanoparticles, including undoped MBGNs and those doped with different Rb concentrations (0Rb, 5Rb, and 10Rb), exhibited consistent spherical and ellipsoidal morphologies with high specific surface area and uniform mesopores and sizes in the range 50–200 nm. Varying Rb concentrations did not affect the amorphous character or morphological properties of the MBGNs. All MBGN compositions demonstrated bioactivity by inducing hydroxycarbonate apatite (HCA) formation. HCA on the pellet surface presented a needle-like structure after 7 days of immersion in SBF. In vitro testing revealed that even at the highest Rb concentration (10Rb), there was no observed toxicity towards MC3T3-E1 pre-osteoblastic cells in the direct exposure method at nanoparticle concentrations up to 250 µg/ml. At concentrations ranging from 50 to 100 µg/ml, 10Rb exhibited the highest level of cell viability. Regarding antibacterial properties, 0Rb pellet showed no bacterial inhibition zone for E. coli and S. aureus. Interestingly, 5Rb and 10Rb samples significantly enhanced the antibacterial activity of MBGNs against both E. coli and S. aureus, producing an inhibition zone. Therefore, the physicochemical and biological properties of 10Rb indicated that these nanoparticles can be a promising functional bio-filler for bone tissue regeneration applications. Future work will focus on investigating the osteogenic and angiogenic in vitro activity of Rb-MBG nanoparticles.
Data Availability
Datasets can be obtained upon request from the corresponding author.
References
L. Chen, X. Zhou, C. He, Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 11(6), 1–22 (2019). https://doi.org/10.1002/wnan.1573
A. López-Noriega, D. Arcos, I. Izquierdo-Barba, Y. Sakamoto, O. Terasaki, M. Vallet-Regí, Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 18(13), 3137–3144 (2006). https://doi.org/10.1021/cm060488o
X. Yan, C. Yu, X. Zhou, J. Tang, D. Zhao, Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chemie - Int. Ed. 43(44), 5980–5984 (2004). https://doi.org/10.1002/anie.200460598
N. Gupta, D. Santhiya, Mesoporous Bioactive Glass and its Applications (Elsevier Ltd., Second Edi, 2018)
C. Wu, J. Chang, Mesoporous bioactive glasses: structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus. 2(3), 292–306 (2012). https://doi.org/10.1098/rsfs.2011.0121
S. Kargozar, M. Montazerian, S. Hamzehlou, H.-W. Kim, F. Baino, Mesoporous bioactive glasses (MBGs): promising platforms for antibacterial strategies Saeid. Acta Biomater. 81, 1–19 (Nov. 2018). https://doi.org/10.1016/j.actbio.2018.09.052
K. Deshmukh, T. Kovářík, T. Křenek, D. Docheva, T. Stich, J. Pola, Recent advances and future perspectives of sol-gel derived porous bioactive glasses: a review. RSC Adv. 10(56), 33782–33835 (2020). https://doi.org/10.1039/d0ra04287k
S. Sanchez-Salcedo et al., Highly-bioreactive silica-based mesoporous bioactive glasses enriched with gallium(III). Mater. (Basel). 11(3), 1–17 (2018). https://doi.org/10.3390/ma11030367
Q. Liang, Q. Hu, G. Miao, B. Yuan, X. Chen, A facile synthesis of novel mesoporous bioactive glass nanoparticles with various morphologies and tunable mesostructure by sacrificial liquid template method. Mater. Lett. 148, 45–49 (2015). https://doi.org/10.1016/j.matlet.2015.01.122
C. Wu, J. Chang, Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control Release. 193, 282–295 (2014). https://doi.org/10.1016/j.jconrel.2014.04.026
I. Izquierdo-Barba, M. Vallet-Regi, Mesoporous bioactive glasses: relevance of their porous structure compared to that of classical bioglasses. Biomed. Glas. 1(1), 140–150 (2015). https://doi.org/10.1515/bglass-2015-0014
A. Bari et al., Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 55, 493–504 (2017). https://doi.org/10.1016/j.actbio.2017.04.012
Z. Neščáková et al., Multifunctional zinc ion doped sol – gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 4, 312–321 (2019). https://doi.org/10.1016/j.bioactmat.2019.10.002
R.F. Richter, T. Ahlfeld, M. Gelinsky, A. Lode, Composites consisting of calcium phosphate cements and mesoporous bioactive glasses as a 3D plottable drug delivery system. Acta Biomater. 156, 146–157 (2023). https://doi.org/10.1016/j.actbio.2022.01.034
F. Kermani et al., Zinc- and copper-doped Mesoporous Borate Bioactive glasses: Promising additives for potential use in skin wound Healing Applications. Int. J. Mol. Sci. 24(2), 1304 (2023). https://doi.org/10.3390/ijms24021304
S. Sánchez-Salcedo, A. García, A. González-Jiménez, M. Vallet-Regí, Antibacterial effect of 3D printed mesoporous bioactive glass scaffolds doped with metallic silver nanoparticles. Acta Biomater. 155, 654–666 (2023). https://doi.org/10.1016/j.actbio.2022.10.045
D. Kozon, K. Zheng, E. Boccardi, Y. Liu, L. Liverani, A.R. Boccaccini, Synthesis of monodispersed Ag-doped bioactive glass nanoparticles via surface modification. Mater. (Basel). 9(4), 225 (2016). https://doi.org/10.3390/ma9040225
M. Hosseini et al., November., Facile post modification synthesis of copper-doped mesoporous bioactive glass with high antibacterial performance to fight bone infection. Biomater. Adv. 144, 213198 (2023). https://doi.org/10.1016/j.bioadv.2022.213198
U. Pantulap, M. Arango-Ospina, A.R. Boccaccini, Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 33(1) (2022). https://doi.org/10.1007/s10856-021-06626-3
V. Zaichick, Chemical elements of human bone tissue investigated by nuclear analytical and related methods. Biol. Trace Elem. Res. 153, 1–3 (2013). https://doi.org/10.1007/s12011-013-9661-4
V. Zaichick, S. Zaichick, Twenty Chemical element contents in normal and cancerous thyroid. Int. J. Hematol. Blood Disord. 3(2), 1–13 (2018). https://doi.org/10.15226/ijhbd/3/2/00121
F.H. Nielsen. Ultratrace elements in nutrition: Current knowledge and speculation. J. Trace Elem. Exp. Med. 11, 251–274 (1998). https://doi.org/10.1002/(SICI)1520-670X(1998)11:2/3<251::AID-JTRA15>3.0.CO;2-Q
M. Chen, S. Wu, Y. Tan, R. Li, Y. Liu, Q. Huang, Rubidium-doped titanium surfaces with modulatory effects on MC3T3-E1 cell response and antibacterial capacity against Staphylococcus aureus. Biomed. Mater. 14, 045016 (2019). https://doi.org/10.1088/1748-605X/ab2585
Z. Ouyang, Q. Huang, B. Liu, H. Wu, T. Liu, Y. Liu. Rubidium chloride targets JNK/p38-mediated NF-κB activation to attenuate osteoclastogenesis and facilitate osteoblastogenesis. Front. Pharmacol. 10, 1–12 (2019). https://doi.org/10.3389/fphar.2019.00584
Y. Liu, Y. Tan, J. Wu. Rubidium doped nano-hydroxyapatite with cytocompatibility and antibacterial. J. Asian Ceram. Soc. 9(1), 323–333 (2021). https://doi.org/10.1080/21870764.2020.1865861
S. Ouyang, K. Zheng, Q. Huang, Y. Liu, A.R. Boccaccini, Synthesis and characterization of rubidium-containing bioactive glass nanoparticles. Mater. Lett. 273, 127920 (2020). https://doi.org/10.1016/j.matlet.2020.127920
Y. ni Tan, W. Chen, W. Wei, Q. li Huang, X. He. Rubidium-modified bioactive glass-ceramics with hydroxyapatite crystals for bone regeneration. Trans. Nonferrous Met. Soc. China. 31(2), 521–532 (2021). https://doi.org/10.1016/S1003-6326(21)65514-0
A. Ruiz-Clavijo, A.P. Hurt, A.K. Kotha, N.J. Coleman, Effect of calcium precursor on the bioactivity and biocompatibility of sol-gel-derived glasses. J. Funct. Biomater. 10(1), 1–16 (2019). https://doi.org/10.3390/jfb10010013
Q. Hu et al. The effects of morphology on physicochemical properties, bioactivity and biocompatibility of micro-/nano-bioactive glasses. Adv. Powder Technol. 29, 1812–1819 (2018). https://doi.org/10.1016/j.apt.2018.04.017
T. Kokubo, H. Takadama, How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 27(15), 2907–2915 (2006). https://doi.org/10.1016/j.biomaterials.2006.01.017
ISO - ISO 10993-, 5:2009 - Biological evaluation of medical devices — part 5: tests for in vitro cytotoxicity. https://www.iso.org/standard/36406.html (accessed Mar. 08, 2023)
I. Unalan, T. Fuggerer, B. Slavik, A. Buettner, A.R. Boccaccini. Antibacterial and antioxidant activity of cinnamon essential oil-laden 45S5 bioactive glass/soy protein composite scaffolds for the treatment of bone infections and oxidative stress. Mater. Sci. Eng. C. 128, 112320 (2021). https://doi.org/10.1016/j.msec.2021.112320
F. Vergnaud, B. Mekonnen, A. El Abbassi, C. Vichery, J.-M. Nedelec. Correlating the effect of composition and textural properties on bioactivity for pristine and copper-doped binary mesoporous bioactive glass nanoparticles. Materials. 16, 6690 (2023). https://doi.org/10.3390/ma16206690
B.R. Barrioni et al., Effects of manganese incorporation on the morphology, structure and cytotoxicity of spherical bioactive glass nanoparticles. J. Colloid Interface Sci. 547, 382–392 (2019). https://doi.org/10.1016/j.jcis.2019.04.016
A. Pinna et al., Acta Biomaterialia Nanoceria provides antioxidant and osteogenic properties to mesoporous silica nanoparticles for osteoporosis treatment. Acta Biomater. 122, 365–376 (2021). https://doi.org/10.1016/j.actbio.2020.12.029
K. Deepa, V. Jaisankar. A facile synthesis and characterisation of novel mesoporous bioactive glass nanoparticles/Xylitol based biodegradable polyester composites for in vitro degradation studies and anti-inflammatory activity. Mater. Today Proc. 14, 461–470 (2019). https://doi.org/10.1016/j.matpr.2019.04.169
L. Qu et al. A signal-off electrochemical sensing platform based on Fe3S4-Pd and pineal mesoporous bioactive glass for procalcitonin detection. Sens. Actuators B Chem. 320, 128324 (2020). https://doi.org/10.1016/j.snb.2020.128324
A. Polo-montalvo, L. Casarrubios. Effective actions of ion release from mesoporous bioactive glass and macrophage mediators on the differentiation of osteoprogenitor and endothelial progenitor cells. Pharmaceutics. 13, 1152 (2021). https://doi.org/10.3390/pharmaceutics13081152
F. Westhauser, S. Wilkesmann, Q. Nawaz, S.I. Schmitz, A. Moghaddam, A.R. Boccaccini, osteogenic properties of manganese-doped mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. Part A. 108, 1806–1815 (2020). https://doi.org/10.1002/jbm.a.36945
X. Wang, Y. Zhang, Y. Ma, D. Chen, H. Yang, M. Li. Selenium – containing mesoporous bioactive glass particles: Physicochemical and drug delivery properties. Ceram. Int. 42(2), 3609–3617 (2016). https://doi.org/10.1016/j.ceramint.2015.11.024
F.E. Ciraldo, L. Liverani, L. Gritsch, W.H. Goldmann, A.R. Boccaccini, Synthesis and characterization of silver-doped mesoporous bioactive glass and its applications in conjunction with electrospinning. Mater. (Basel). 11(5) (2018). https://doi.org/10.3390/ma11050692
X. Wang et al., A pH-neutral bioactive glass coated 3D-printed porous Ti6Al4V scaffold with enhanced osseointegration. J. Mater. Chem. B 11(6), 1203–1212 (2023). https://doi.org/10.1039/D2TB02129C
I.R. de Oliveira et al. Biocomposite macrospheres based on strontium-bioactive glass for application as bone fillers. ACS Mater. Au. 3, 646–658 (2023). https://doi.org/10.1021/acsmaterialsau.3c00048
J. Liu, G. Du, H. Yu, X. Zhang, T. Chen. Synthesis of hierarchically porous Bioactive Glass and its mineralization activity. Molecules. 28(5) 2224 (2023). https://doi.org/10.3390/molecules28052224
R. Wetzel, D.S. Brauer, Apatite formation of substituted Bioglass 45S5: SBF vs. Tris. Mater. Lett. 257, 126760 (2019). https://doi.org/10.1016/j.matlet.2019.126760
B.R. Barrioni, E. Norris, S. Li, P. Naruphontjirakul, J.R. Jones, M.M. Pereira. Osteogenic potential of sol–gel bioactive glasses containing manganese. J. Mater. Sci. Mater. Med. 30, 86 (2019). https://doi.org/10.1007/s10856-019-6288-9
J.E. Gough, J.R. Jones, L.L. Hench, Nodule formation and mineralisation of human primary osteoblasts cultured on a porous bioactive glass scaffold. Biomaterials. 25(11), 2039–2046 (2004). https://doi.org/10.1016/j.biomaterials.2003.07.001
A.M. Galow, A. Rebl, D. Koczan, S.M. Bonk, W. Baumann, J. Gimsa. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem. Biophys. Reports. 10, 17–25 (2017). https://doi.org/10.1016/j.bbrep.2017.02.001
P. Naruphontjirakul, A.E. Porter, J.R. Jones. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 66, 67–80 (2018). https://doi.org/10.1016/j.actbio.2017.11.008
J.E. Hellwinkel, Z.M. Working, L. Certain, A.J. García, J.C. Wenke, C.S. Bahney. The intersection of fracture healing and infection: Orthopaedics research society workshop 2021. J. Orthop. Res. 40, 541–552 (2022). https://doi.org/10.1002/jor.25261
M.S.K. Mubina, S. Shailajha, R. Sankaranarayanan, L. Saranya. In vitro bioactivity, mechanical behavior and antibacterial properties of mesoporous SiO2-CaO-Na2O-P2O5 nano bioactive glass ceramics. J. Mech. Behav. Biomed. Mater. 100, 103379 (2019). https://doi.org/10.1016/j.jmbbm.2019.103379
Acknowledgments
Usanee Pantulap would like to thank the Royal Thai Government Scholarship under the Ministry of Higher Education, Science, Research and Innovation. The authors thank Dr. Hana Kankova (FunGlass - Center for Functional and Surface Funtionalized Glass), Trencin, Slovakia, for carrying out the ICP measurements.
Funding
Royal Thai Government Scholarship under the Ministry of Higher Education, Science, Research and Innovation.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
UP conceptualized th study, carried out most of the experimental work, data analysis, wrote the original draft. IU supported experimental work, reviewed and edited the manuscript. KZ contributed to the discussion of results, reviewed and edited the manuscripüt, ARB supervised and conceptualized the study, provided resources, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pantulap, U., Unalan, I., Zheng, K. et al. Hydroxycarbonate apatite formation, cytotoxicity, and antibacterial properties of rubidium-doped mesoporous bioactive glass nanoparticles. J Porous Mater 31, 685–696 (2024). https://doi.org/10.1007/s10934-023-01546-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-023-01546-9