Abstract
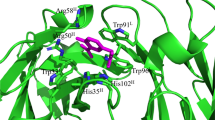
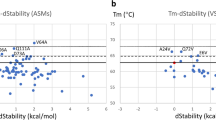
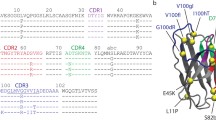
Somatic hypermutation (SHM) is one of the driving forces that increases antibody (Ab) affinity. We studied the effects of SHM on thermostability and affinity using three single-chain Fv fragments (scFvs) of anti-(4-hydroxy-3-nitrophenyl)acetyl Abs, namely 9TG, 9T7, and E11. 9TG has a germline structure that lacks SHM and is an ancestor of 9T7 with 11 mutations. E11, which has 21 mutations, is a mature Ab and has its own ancestor. The thermostabilities and antigen-Ab interactions were analyzed by circular dichroism (CD), differential scanning calorimetry (DSC), and isothermal titration calorimetry (ITC). Far-UV CD spectra showed that all scFvs were folded into a structure referred to as immunoglobulin-fold and were unfolded by heating at different melting temperatures. Comparison of thermodynamic parameters obtained from DSC and ITC revealed that the magnitude of stabilization free energy at 37 °C was in the order, 9TG > 9T7 > E11, while that of the free energy of interaction with antigen was 9TG < 9T7 < E11, suggesting that Abs make a trade-off between stability and affinity during affinity maturation.







Similar content being viewed by others
References
Tonegawa S (1983) Somatic generation of antibody diversity. Nature 302:575–581. https://doi.org/10.1038/302575a0
Alt FW, Baltimore D (1982) Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci USA 79:4118–4122. https://doi.org/10.1073/pnas.79.13.4118
Neuberger MS, Milstein C (1995) Somatic hypermutation. Curr Opin Immunol 7:248–254. https://doi.org/10.1016/0952-7915(95)80010-7
Azuma T (1998) Somatic hypermutation in mouse λ chains. Immunol Rev 162:97–105. https://doi.org/10.1111/j.1600-065x
Eisen HN, Siskind GW (1964) Variation in affinities of antibody during immune response. Biochemistry 3:996–1008. https://doi.org/10.1021/bi00895a027
Griffiths GM, Berek C, Kaartinen M, Milstein C (1984) Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature 312:271–275. https://doi.org/10.1038/312271a0
Milstein C, Rada C (1995) The maturation of the antibody response. In: Honjo T, Alt FW (eds) Immunoglobulin genes, 2nd edn. Academic Press, pp 57–81
Cumano A, Rajewsky K (1985) Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur J Immunol 15:512–520. https://doi.org/10.1002/eji.1830150517
Allen D, Simon T, Sablitzky F, Rajewsky K, Cumano A (1988) Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J 7:1995–2001. https://doi.org/10.1002/j.1460-2075.1988.tb03038
Furukawa K, Akasako-Furukawa A, Shirai H, Nakamura H, Azuma T (1999) Junctional amino acids determine the maturation pathway of an antibody. Immunity 11:329–338. https://doi.org/10.1016/s1074-7613(00)80108-9
Murakami A, Takahashi Y, Nishimura M, Shimizu T, Azuma T (2010) The amino acid residue at position 95 and the third CDR region in the H chain determine the ceiling affinity and the maturation pathway of an anti-(4-hydroxy-3-nitrophenyl)acetyl antibody. Mol Immunol 48:48–58. https://doi.org/10.1016/j.molimm.2010.09.013
Sato Y, Inaba S, Fukada H, Azuma T, Oda M (2017) Pronounced effect of hapten binding on thermal stability of an anti-(4-hydroxy-3-nitrophenyl)acetyl antibody possessing a glycine residue at position 95 of the heavy chain. Mol Immunol 85:130–136. https://doi.org/10.1016/j.molimm.2017.02.010
Ott JA, Castro CD, Deiss TC, Ohta Y, Flajnik MF, Criscitiello MF (2018) Somatic hypermutation of T cell receptor α chain contributes to selection in nurse shark thymus. Elife 7:e28477. https://doi.org/10.7554/eLife.28477
Beadle BM, Shoichet BK (2002) Structural bases of stability-function tradeoffs in enzymes. J Mol Biol 321:285–296. https://doi.org/10.1016/s0022-2836(02)00599-5
Nishimura M, Murakami A, Hara Y, Azuma T (2011) Characterization of memory B cells responsible for affinity maturation of anti- (4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies. Int Immunol 23:271–285. https://doi.org/10.1093/intimm/dxr002
Tashiro Y, Murakami A, Goizuka R, Shimizu T, Kishimoto H, Azuma T (2015) An asymmetric antibody repertoire is shaped between plasmablasts and plasma cells after secondary immunization with (4-hydroxy-3-nitrophenyl)acetyl chicken γ-globulin. Int Immunol 27:609–620. https://doi.org/10.1093/intimm/dxv040
Tashiro Y, Murakami A, Hara Y, Shimizu T, Kubo M, Goitsuka R, Kishimoto H, Azuma T (2018) High-affinity IgM+ memory B cells are defective in differentiation into IgM antibody-secreting cells by re-stimulation with a T cell-dependent antigen. Sci Rep 8:14559. https://doi.org/10.1038/s41598-018-32926-w
Furukawa K, Shimizu T, Murakami A, Kono R, Nakagawa M, Sagawa T, Yamato I, Azuma T (2007) Strategy for affinity maturation of an antibody with high evolvability to (4-hydroxy-3-nitrophenyl)acetyl hapten. Mol Immunol 44:2436–2445. https://doi.org/10.1016/j.molimm.2006.10.023
Taketani M, Naitoh A, Motoyama N, Azuma T (1995) Role of conserved amino acid residues in the complementarity determining regions on hapten-antibody interaction of anti-(4-hydroxy-3-nitrophenyl) acetyl antibodies. Mol Immunol 32:983–990. https://doi.org/10.1016/0161-5890(95)00057-l
Sato Y, Tanaka Y, Inaba S, Sekiguchi H, Maruno T, Sasaki YC, Fukada H, Kobayashi Y, Azuma T, Oda M (2016) Structural dynamics of a single-chain Fv antibody against (4-hydroxy-3-nitrophenyl)acetyl. Int J Biol Macromol 91:151–157. https://doi.org/10.1016/j.ijbiomac.2016.05.074
Oda M, Azuma T (2000) Reevaluation of stoichiometry and affinity/avidity in interactions between anti-hapten antibodies and mono- or multi-valent antigens. Mol Immunol 37:1111–1122. https://doi.org/10.1016/s0161-5890(01)00028-1
Oda M, Azuma T (2016) Affinity maturation of anti-(4-hydroxy-3-nitrophenyl)acetyl antibodies accompanies a modulation of antigen specificity. Mol Immunol 70:8–12. https://doi.org/10.1016/j.molimm.2015.12.003
Inaba S, Fukada H, Ikegami T, Oda M (2013) Thermodynamic effects of multiple protein conformations on stability and DNA binding. Arch Biochem Biophys 537:225–232. https://doi.org/10.1016/j.abb.2013.07.014
Tadokoro T, Kazama H, Koga Y, Takano K, Kanaya S (2013) Investigating the structural dependence of protein stabilization by amino acid substitution. Biochemistry 52:2839–2847. https://doi.org/10.1021/bi400076f
Fukada H, Sturtevant JM, Quiocho FA (1983) Thermodynamics of the binding of L-arabinose and of D-galactose to the L-arabinose-binding protein of Escherichia coli. J Biol Chem 258:13193–13198. https://doi.org/10.1016/S0021-9258(17)44100-7
Sagawa T, Oda M, Ishimura M, Furukawa K, Azuma T (2003) Thermodynamic and kinetic aspects of antibody evolution during the immune response to hapten. Mol Immunol 39:801–808. https://doi.org/10.1016/s0161-5890(02)00282-1
Mizutani R, Miura K, Nakayama T, Shimada I, Arata Y, Satow Y (1995) Three dimensional structures of the Fab fragment of murine N1G9 antibody from the primary immune response and of its complex with (4-hydroxy-3-nitrophenyl)acetate. J Mol Biol 254:208–222. https://doi.org/10.1006/jmbi.1995.0612
Nishiguchi A, Numoto N, Ito N, Azuma T, Oda M (2019) Three-dimensional structure of a high affinity anti-(4-hydroxy-3-nitrophenyl)acetyl antibody possessing a glycine residue at position 95 of the heavy chain. Mol Immunol 114:545–552. https://doi.org/10.1016/j.molimm.2019.09.001
Brandts JF, Lin LN (1990) Study of strong to ultratight protein interactions using differential scanning calorimetry. Biochemistry 29:6927–6940. https://doi.org/10.1021/bi00481a024
Matulis D, Kranz JK, Salemme FR, Todd MJ (2005) Thermodynamic stability of carbonic anhydrase: measurements of binding affinity and stoichiometry using ThermoFluor. Biochemistry 44:5258–5266. https://doi.org/10.1021/bi048135v
Vuorinen E, Valtonen S, Eskonen V, Kariniemi T, Jakovleva J, Kopra K, Härmä H (2020) Sensitive label-free thermal stability assay for protein denaturation and protein-ligand interaction studies. Anal Chem 92:3512–3516. https://doi.org/10.1021/acs.analchem.9b05712
Yin J, Beuscher AE 4th, Andryski SE, Stevens RC, Schultz PG (2003) Structural plasticity and the evolution of antibody affinity and specificity. J Mol Biol 330:651–656. https://doi.org/10.1016/s0022-2836(03)00631-4
Acknowledgements
The authors thank to Dr. Harumi Fukada for helpful discussion. This work was performed in part under the Collaborative Research Program of Institute for Protein Research, Osaka University, CR-20-02, and was partly supported by Nanotechnology Platform Program <Molecule and Material Synthesis> (JPMXP09S21MS1006) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This study was supported by JSPS KAKENHI Grant Number 18K06161.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishiguchi, A., Murakami, A., Azuma, T. et al. A Trade-off Between Thermostability and Binding Affinity of Anti-(4-hydroxy-3-nitrophenyl)Acetyl Antibodies During the Course of Affinity Maturation. Protein J 41, 293–303 (2022). https://doi.org/10.1007/s10930-022-10053-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-022-10053-w