Abstract
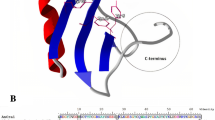
In this study, the role of two conversed tyrosines (Tyr5 and Tyr42) from the scorpion toxin BmK AGP-SYPU1 was investigated with an effective Escherichia coli expression system. Site-directed mutagenesis was used to individually substitute Tyr5 and Tyr42 with hydrophobic or hydrophilic amino acids, and the extent to which these scorpion toxin BmK AGP-SYPU1 tyrosines contribute to analgesic activity was evaluated. The results of the mouse-twisting test showed that Tyr5 and Tyr42 are associated with the analgesic activity of the toxin because the analgesic activities of Y5F and Y42F were significantly increased compared with the rBmK AGP-SYPU1; however, the Y5W had decreased activity. The results of molecular simulation reveal the following: (1) for analgesic activity, the core domain of the scorpion toxin BmK AGP-SYPU1 is key and (2) for pharmacological function, Tyr42 is most likely involved when the core domain conformation is altered. These studies identify a new relationship between the structure and analgesic activity of the scorpion toxin BmK AGP-SYPU1 and are significant for further research and the application of analgesic peptides.





Similar content being viewed by others
Abbreviations
- BmK:
-
Buthus martensii Karsch
- BmK AGP-SYPU1:
-
Analgesic peptide 1 from Buthus martensii Karsch by Shenyang Pharmaceutical University
- rBmK AGP-SYPU1:
-
Recombinant BmK AGP-SYPU1
- Lqq:
-
Leiurus quinquestriatus quinquestriatus
- E. coli :
-
Escherichia coli
- PCR:
-
Polymerase chain reaction
- 3D:
-
Three-dimensional
- SDS:
-
Sodium dodecyl sulfate
- PAGE:
-
Polyacrylamide gel electrophoresis
References
Ma R, Cui Y, Zhou Y, Bao YM, Yang WY, Liu YF, Wu CF, Zhang JH (2010) Location of the analgesic domain in Scorpion toxin BmK AGAP by mutagenesis of disulfide bridges. Biochem Biophys Res Commun 394:330–334
Xiong YM, Lan ZD, Wang M, Liu B, Liu XQ, Fei H, Xu LG, Xia QC, Wang CG, Wang DC, Chi CW (1999) Molecular characterization of a new excitatory insect neurotoxin with an analgesic effect on mice from the scorpion Buthus martensi Karsch. Toxicon 37:1165–1180
Ji YH, Li YJ, Zhang JW, Song BL, Yamaki T, Mochizuki T, Hoshino M, Yanaihara N (1999) Covalent structures of BmK AS and BmK AS-1, two novel bioactive polypeptides purified from Chinese scorpion Buthus martensii Karsch. Toxicon 37:519–536
Wang CY, Tan ZY, Chen B, Zhao ZQ, Ji YH (2000) Antihyperalgesia effect of BmK IT2, a depressant insect-selective scorpion toxin in rat by peripheral administration antihyperalgesia. Brain Res Bull 53:335–338
Guan R, Wang CG, Wang M, Wang DC (2001) A depressant insect toxin with a novel analgesic effect from scorpion Buthus martensii Karsch. Biochim Biophys Acta 1549:9–18
Cao ZY, Mi ZM, Cheng GF, Shen WQ, Xiao X, Liu XM, Liang XT, Yu DQ (2004) Purification and characterization of a new peptide with analgesic effect from the scorpion Buthus martensii Karsch. J Pept Res 64:33–41
Wang Y, Wang L, Cui Y, Song YB, Liu YF, Zhang R, Wu CF, Zhang JH (2011) Purification, characterization and functional expression of a new peptide with an analgesic effect from Chinese scorpion Buthus martensii Karsch (BmK AGP-SYPU1). Biomed Chromatogr 25:801–807
Zhang R, Cui Y, Zhang X, Yang Z, Zhao Y, Song Y, Wu C, Zhang J (2010) Soluble expression, purification and the role of C-terminal glycine residues in scorpion toxin BmK AGP-SYPU2. BMB Rep 43:801–806
Shao JH, Cui Y, Zhao MY, Wu CF, Liu YF, Zhang JH (2013) Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides. doi: 10.1016/j.peptides.2013.10.023
Wang YQ, Hao Z, Shao J, Song Y, Li C, Li C, Zhao Y, Liu Y, Wei T, Wu C, Zhang J (2011) The role of Ser54 in the antinociceptive activity of BmK9, a neurotoxin from the scorpion Buthus martensii Karsch. Toxicon 58:527–532
Mouhat S, Jouirou B, Mosbah A, De Waard M, Sabatier JM (2004) Diversity of folds in animal toxins acting on ion channels. Biochem J 378:717–726
Gurevitz M, Karbat I, Cohen L, Ilan N, Kahn R, Turkov M, Stankiewicz M, Stuhmer W, Dong K, Gordon D (2007) The insecticidal potential of scorpion beta-toxins. Toxicon 49:473–489
Gordon D, Karbat I, Ilan N, Cohen L, Kahn R, Gilles N, Dong K, Stuhmer W, Tytgat J, Gurevitz M (2007) The differential preference of scorpion alpha-toxins for insect or mammalian sodium channels: implications for improved insect control. Toxicon 49:452–472
Tong X, Yao J, He F, Chen X, Zheng X, Xie C, Wu G, Zhang N, Ding J, Wu H (2006) NMR solution structure of BmK-betaIT, an excitatory scorpion beta-toxin without a ‘hot spot’ at the relevant position. Biochem Biophys Res Commun 349:890–899
Cui Y, Guo GL, Ma L, Hu N, Song YB, Liu YF, Wu CF, Zhang JH (2010) Structure and function relationship of toxin from Chinese scorpion Buthus martensii Karsch (BmK AGAP): gaining insight into related sites of analgesic activity. Peptides 31:995–1000
Márcia RM, Alexandra OSC (2013) Gandhi RB (ed) New Perspectives in drug discovery using neuroactive molecules from the venom of arthropods: an integrated view of the molecular recognition and toxinology—from analytical procedures to biomedical applications. ISBN: 978-953-51-1151-1, InTech. doi:10.5772/52382
Wang Y, Song YB, Yang GZ, Cui Y, Zhao YS, Liu YF, Ma Y, Wu CF, Zhang JH (2012) Arginine residues in the C-terminal and their relationship with the analgesic activity of the toxin from the Chinese scorpion Buthus martensii Karsch (BmK AGP-SYPU1). Appl Biochem Biotechnol 168:247–255
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Fennessy MR, Lee JR (1975) Methods in narcotics research. In: Ehrenpreis S, Neidle A (eds) Modern pharmacology-toxicology. Marcel Dekker, NewYork, pp 76–79
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291
Shapiro AL, Viñuela E, Maizel V Jr (1967) Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun 28:815–820
Kopeyan C, Mansuelle P, Martin-Eauclaire MF, Rochat H, Miranda F (1993) Characterization of toxin III of the scorpion Leiurus quinquestriatus quinquestriatus: a new type of alpha-toxin highly toxic both to mammals and insects. Nat Toxins 1:308–312
Landon C, Cornet B, Bonmatin JM, Kopeyan C, Rochat H, Vovelle F, Ptak M (1996) 1H-NMR-derived secondary structure and the overall fold of the potent anti-mammal and anti-insect toxin III from the scorpion Leiurus quinquestriatus quinquestriatus. Eur J Biochem 236:395–404
He XL, Li HM, Zeng ZH, Liu XQ, Wang M, Wang DC (1999) Crystal structures of two alpha-like scorpion toxins: non-proline cis peptide bonds and implications for new binding site selectivity on the sodium channel. J Mol Biol 292:125–135
Sun YM, Liu W, Zhu RH, Goudet C, Tytgat J, Wang DC (2002) Roles of disulfide bridges in scorpion toxin BmK M1 analyzed by mutagenesis. J Pept Res 60:247–256
Sun YM, Bosmans F, Zhu RH, Goudet C, Xiong YM, Tytgat J, Wang DC (2003) Importance of the conserved aromatic residues in the scorpion alpha-like toxin BmK M1: the hydrophobic surface region revisited. J Biol Chem 278:24125–24131
Xiong YM, Ling MH, Wang DC, Chi CW (1997) The CDNA and genomic DNA sequences of a mammalian neurotoxin from the scorpion Buthus martensii Karsch. Toxicon 35:1025–1031
Acknowledgments
This study was supported by the Grants from the National Natural Science Foundation of China (81072568), Major Drug Innovation of National Eleventh Five-Year Major Project of China (2009ZX09301-012), the Natural Science Foundation of China (81102365), and Specialized Research Fund for the Doctoral Program of Higher Education (20112134120008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, L., Zhang, HX., Wang, Y. et al. Site-Directed Mutagenesis of BmK AGP-SYPU1: The Role of Two Conserved Tyr (Tyr5 and Tyr42) in Analgesic Activity. Protein J 33, 157–164 (2014). https://doi.org/10.1007/s10930-014-9547-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-014-9547-0