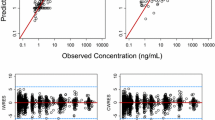
Abstract
Danvatirsen is a Generation 2.5 antisense oligonucleotide under clinical development. Population PK modelling was conducted using data from 3 available danvatirsen Phase I/II studies in oncology patients to investigate the impact of flat dosing on exposure compared to ideal body weight-based dosing. A total of 126 patients who received danvatirsen doses ranging from 1 to 4 mg/kg as monotherapy or in combination with durvalumab, most at 3 mg/kg (n = 70), was used in the danvatirsen population PK analysis. A 2-compartment model with linear elimination described the data well. Covariate analysis revealed ideal body weight was not a significant covariate on the PK of danvatirsen; nor was age, sex or race. The model-based simulation suggested that steady state weekly AUC and Cmax were very similar between 3 mg/kg and 200 mg flat dosing (geometric mean of AUC: 62.5 vs. 63.4 mg h/L and Cmax: 26.2 vs. 26.5 mg/L for two dose groups) with slightly less overall between-subject variability in the flat dosing regimen. The switch to flat dosing was approved by multiple regulatory agencies, including FDA, EMA, PMDA and ANSM. Several ongoing studies have been evaluating flat dosing. Interim analysis from an ongoing study (D5660C00016, NCT03421353) has shown the observed steady state concentration from 200 mg flat dose is in agreement with the model predictions. The population PK model could be further utilized in subsequent exposure-response efficacy and safety modelling.






Similar content being viewed by others
References
Crooke ST, Bennett CF (1996) Progress in antisense oligonucleotide therapeutics. Annu Rev Pharmacol Toxicol 36:107–129
Monia BP, Lesnik EA, Gonzalez Lima WF, McGee D, Guinosso CJ, Kawasaki AM, Cook PD, Freier SM (1993) Evaluation of 2′-modified oligonucleotides containing 2′-deoxy gaps as antisense inhibitors of gene expression. J Biol Chem 268:14514–14522
Seth PP, Siwkowski A, Allerson CR, Vasquez G, Lee S, Prakash TP, Wancewicz EV, Witchell D, Swayze EE (2009) Short antisense oligonucleotides with novel 2′-4′ conformationaly restricted nucleoside analogues show improved potency without increased toxicity in animals. J Med Chem 52(1):10–13
Yu RZ, Grundy JS, Geary RS (2013) Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert Opin Drug Metab Toxicol 9(2):169–182
Food and Drug Administration (2001) USFDA. Guidance for industry: bioanalytical method validation, vol 66. https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N et al (2017) Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol 6(2):87–109
Tong X, Zhou D, Savage A, Mullen JA, Li Y, Taylor W, Li J, Al-Huniti N, Xu H (2018) Population pharmacokinetic modeling with enterohepatic circulation for AZD3241 in healthy subjects and patients with multiple system atrophy. J Clin Pharmacol 58(11):1452–1460
Zhou W, Li L, Birmingham B, Xu H, Lillieborg S, Zhou D, Al-Huniti N (2017) Population pharmacokinetic analysis of zolmitriptan and its metabolite in adults and adolescents to support dose selection in children with migraine. J Clin Pharmacol 57(10):1258–1267
Xu H, Li J, Webber L, Kakkar R, Chen Y, Al-Huniti N (2016) Population pharmacokinetic and pharmacodynamic modeling of azd4901 and simulation to support dose selection for the phase 2a study. J Clin Pharmacol 56(8):999–1008
Al-Huniti N, Xu H, Zhou D, Aksenov S, Fox R, Bui KH (2017) Population exposure-response modeling supported selection of naloxegol doses in phase III studies in patients with opioid-induced constipation. CPT Pharmacometrics Syst Pharmacol 6(10):705–711. https://doi.org/10.1002/psp4.12229
Al-Huniti N, Zhou D, Xu H, Aksenov S, Bui KH, Fox R, Helmlinger G, Stanski D (2017) Pharmacometric modeling of naloxegol efficacy and safety: impact on dose and label. Clin Pharmacol Ther 102(5):741–744. https://doi.org/10.1002/cpt.719
Zhou D, Li L, Bui K, Learoyd M, Berges A, Milenkova T, Al-Huniti N, Tomkinson H, Xu H (2018) Bridging olaparib capsule and tablet formulations using -population pharmacokinetic meta-analysis in oncology patients. Clin Pharmacokinet 10:100. https://doi.org/10.1007/s40262-018-0714-x
Zhou D, Lu Z, Sunzel M, Xu H, Al-Huniti N (2014) Population pharmacokinetic modelling to assess clinical drug-drug interaction between AZD7325 and midazolam. J Clin Pharm Ther 39(4):404–410
Edwards AY, Elgart A, Farrell C, Barnett-Griness O, Rabinovich-Guilatt L, Spiegelstein O (2017) A population pharmacokinetic meta-analysis of custirsen, an antisense oligonucleotide, in oncology patients and healthy subjects. Br J Clin Pharmacol 83(9):1932–1943
Li Z, Hard ML, Andersen G, Pabst G, Wagener G, Singh T, Chin W, Culm-Merdek K, Boltje I, von Moltke LL (2014) Pharmacokinetics, safety and tolerability of mipomersen in healthy japanese volunteers and comparison with western subjects. Int J Clin Pharmacol Ther 52(4):314–320
Mipomersen (Kynamro) [Package Inert] (2013) Genzyme Corporation. Cambridge. Accessed from https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203568s000lbl.pdf
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, H., Tong, X., Mugundu, G. et al. Population pharmacokinetic analysis of danvatirsen supporting flat dosing switch. J Pharmacokinet Pharmacodyn 46, 65–74 (2019). https://doi.org/10.1007/s10928-019-09619-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-019-09619-6