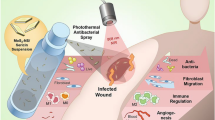
Abstract
Polyhydroxyurethane (PHU) with free secondary amine groups was synthesized by reacting poly(ethylene glycol)bis-cyclic carbonate with triethylenetetramine. PHU was mixed with varying amounts of gelatin (GE) and crosslinked by reacting them with either poly(ethylene glycol)glycidyl ether (PEGDE) or 1,4-butanediol diglycidyl ether (BDDE). The tensile strength and fluid handling capacity of the monolayer films obtained from different formulations of PHU, GE, PEGDE, or BDDE were evaluated. To further improve the tensile strength of dressings via increasing the crosslinking of the networks, varying amounts of epoxidized graphene oxide (EGO) were utilized as an auxiliary crosslinking agent. The improved tensile strength of up to 140% was recorded for these samples. Additionally, the membranes containing EGO were able to absorb near-infrared light. The resulting hyperthermia effect (increasing temperature up to about 63 within 15 min) could efficiently kill bacteria (100% killing). The free secondary amine groups on the PHU backbone also reduced the silver ions loaded into the dressings, and resulting silver nanoparticles (Ag NPs) showed acceptable antibacterial activity against E.coli (53% killing) and S.aureus (78% killing). It was also found that the antibacterial activity of AgNPs-containing samples was further improved after incorporating EGO due to the knife-killing effect of EGO nanoplates.













Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Falanga V, Isseroff RR, Soulika AM et al (2022) Chronic wounds. Nat Rev Dis Prim 8:50. https://doi.org/10.1038/s41572-022-00377-3
Metcalf D, Bowler P (2013) Biofilm delays wound healing: a review of the evidence. Burn Trauma 1:5. https://doi.org/10.4103/2321-3868.113329
Simões D, Miguel SP, Ribeiro MP et al (2018) Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm 127:130–141. https://doi.org/10.1016/j.ejpb.2018.02.022
Liang Y, Liang Y, Zhang H, Guo B (2022) Antibacterial biomaterials for skin wound dressing. Asian J Pharm Sci 17:353–384. https://doi.org/10.1016/j.ajps.2022.01.001
Gholami H, Yeganeh H, Burujeny SB et al (2018) Vegetable oil based polyurethane containing 1,2,3-triazolium functional groups as antimicrobial wound dressing. J Polym Environ 26:462–473. https://doi.org/10.1007/s10924-017-0964-y
Shams E, Yeganeh H, Naderi-Manesh H et al (2017) Polyurethane/siloxane membranes containing graphene oxide nanoplatelets as antimicrobial wound dressings: in vitro and in vivo evaluations. J Mater Sci Mater Med 28:75. https://doi.org/10.1007/s10856-017-5881-z
Gholami H, Yeganeh H (2020) Vegetable oil-based polyurethanes as antimicrobial wound dressings: in vitro and in vivo evaluation. Biomed Mater 15:045001. https://doi.org/10.1088/1748-605X/ab7387
Homaeigohar S, Boccaccini AR (2020) Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater 107:25–49. https://doi.org/10.1016/j.actbio.2020.02.022
Howell-Jones RS, Wilson MJ, Hill KE et al (2005) A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother 55:143–149. https://doi.org/10.1093/jac/dkh513
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249. https://doi.org/10.2147/IJN.S121956
Hajipour MJ, Fromm KM, Akbar Ashkarran A et al (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30:499–511. https://doi.org/10.1016/j.tibtech.2012.06.004
Franci G, Falanga A, Galdiero S et al (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20:8856–8874. https://doi.org/10.3390/molecules20058856
Paladini F, Pollini M (2019) Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials (Basel) 12:2540. https://doi.org/10.3390/ma12162540
Nie P, Zhao Y, Xu H (2023) Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: a review. Ecotoxicol Environ Saf 253:114636. https://doi.org/10.1016/j.ecoenv.2023.114636
Ellison J, Wykoff G, Paul A et al (2014) Efficient dispersion of coated silver nanoparticles in the polymer matrix. Colloids Surf A 447:67–70. https://doi.org/10.1016/j.colsurfa.2014.01.071
Abdali Z, Yeganeh H, Solouk A et al (2015) Thermoresponsive antimicrobial wound dressings via simultaneous thiol-ene polymerization and in situ generation of silver nanoparticles. RSC Adv 5:66024–66036. https://doi.org/10.1039/C5RA11618J
Gharibi R, Yeganeh H, Abdali Z (2018) Preparation of antimicrobial wound dressings via thiol-ene photopolymerization reaction. J Mater Sci 53:1581–1595. https://doi.org/10.1007/s10853-017-1622-4
Rabiee T, Yeganeh H, Gharibi R (2019) Antimicrobial wound dressings with high mechanical conformability prepared through thiol-yne click photopolymerization reaction. Biomed Mater 14:045007. https://doi.org/10.1088/1748-605X/ab16b8
Gharibi R, Yeganeh H, Gholami H, Hassan ZM (2014) Aniline tetramer embedded polyurethane/siloxane membranes and their corresponding nanosilver composites as intelligent wound dressing materials. RSC Adv 4:62046–62060. https://doi.org/10.1039/C4RA11454J
Kim H, Lee YR, Jeong H et al (2023) Photodynamic and photothermal therapies for bacterial infection treatment. Smart Mol. https://doi.org/10.1002/smo.20220010
Chen Y, Gao Y, Chen Y et al (2020) Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release 328:251–262. https://doi.org/10.1016/j.jconrel.2020.08.055
Xu J-W, Yao K, Xu Z-K (2019) Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale 11:8680–8691. https://doi.org/10.1039/C9NR01833F
Jia X, Ahmad I, Yang R, Wang C (2017) Versatile graphene-based photothermal nanocomposites for effectively capturing and killing bacteria, and for destroying bacterial biofilms. J Mater Chem B 5:2459–2467. https://doi.org/10.1039/c6tb03084j
Chao Y, Yu S, Zhang H et al (2023) Architecting lignin/poly(vinyl alcohol) hydrogel with carbon nanotubes for photothermal antibacterial therapy. ACS Appl Bio Mater 6:1525–1535. https://doi.org/10.1021/acsabm.2c01061
Xu X, Ding Y, Hadianamrei R et al (2022) Antimicrobial peptide functionalized gold nanorods combining near-infrared photothermal therapy for effective wound healing. Colloids Surf B 220:112887. https://doi.org/10.1016/j.colsurfb.2022.112887
D’Agostino A, Taglietti A, Desando R et al (2017) Bulk surfaces coated with triangular silver nanoplates: antibacterial action based on silver release and photo-thermal effect. Nanomaterials 7:7. https://doi.org/10.3390/nano7010007
Mutalik C, Okoro G, Krisnawati DI et al (2022) Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J Colloid Interface Sci 607:1825–1835. https://doi.org/10.1016/j.jcis.2021.10.019
Yin W, Yu J, Lv F et al (2016) Functionalized nano-MoS 2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano 10:11000–11011. https://doi.org/10.1021/acsnano.6b05810
Pang Q, Wu K, Jiang Z et al (2022) A polyaniline nanoparticles crosslinked hydrogel with excellent photothermal antibacterial and mechanical properties for wound dressing. Macromol Biosci 22:2100386. https://doi.org/10.1002/mabi.202100386
Zhao Y, Dai X, Wei X et al (2018) Near-infrared light-activated thermosensitive liposomes as efficient agents for photothermal and antibiotic synergistic therapy of bacterial biofilm. ACS Appl Mater Interfaces 10:14426–14437. https://doi.org/10.1021/acsami.8b01327
Wu M-C, Deokar AR, Liao J-H et al (2013) Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano 7:1281–1290. https://doi.org/10.1021/nn304782d
Morales-González M, Díaz LE, Dominguez-Paz C, Valero MF (2022) Insights into the design of polyurethane dressings suitable for the stages of skin wound-healing: a systematic review. Polymers (Basel). https://doi.org/10.3390/polym14152990
Merenyi S (2014) REACH: Regulation (EC) No 1907/2006: Consolidated version (May 2018). GRIN Verlag
Gomez-Lopez A, Elizalde F, Calvo I, Sardon H (2021) Trends in non-isocyanate polyurethane (NIPU) development. Chem Commun 57:12254–12265. https://doi.org/10.1039/D1CC05009E
Chelike DK, Gurusamy Thangavelu SA (2023) Catalyzed and non-catalyzed synthetic approaches to obtain isocyanate-free polyurethanes. ChemistrySelect. https://doi.org/10.1002/slct.202300921
Ecochard Y, Caillol S (2020) Hybrid polyhydroxyurethanes: How to overcome limitations and reach cutting edge properties? Eur Polym J 137:109915. https://doi.org/10.1016/j.eurpolymj.2020.109915
Ke J, Li X, Wang F et al (2016) The hybrid polyhydroxyurethane materials synthesized by a prepolymerization method from CO2-sourced monomer and epoxy. J CO2 Util 16:474–485. https://doi.org/10.1016/j.jcou.2016.11.001
Ke J, Li X, Wang F et al (2017) Non-isocyanate polyurethane/epoxy hybrid materials with different and controlled architectures prepared from a CO 2-sourced monomer and epoxy via an environmentally-friendly route. RSC Adv 7:28841–28852
Ke J, Li X, Jiang S et al (2019) Promising approaches to improve the performances of hybrid non-isocyanate polyurethane. Polym Int 68:651–660. https://doi.org/10.1002/pi.5746
Tudoroiu E-E, Dinu-Pîrvu C-E, Albu Kaya MG et al (2021) An overview of cellulose derivatives-based dressings for wound-healing management. Pharmaceuticals 14:1215. https://doi.org/10.3390/ph14121215
Jayakumar R, Prabaharan M, Sudheesh Kumar PT et al (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29:322–337
Barba BJD, Oyama TG, Taguchi M (2021) Simple fabrication of gelatin–polyvinyl alcohol bilayer hydrogel with wound dressing and nonadhesive duality. Polym Adv Technol 32:4406–4414. https://doi.org/10.1002/pat.5442
Feng P, Luo Y, Ke C et al (2021) Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.650598
Khaloo Kermani P, Zargar Kharazi A (2023) A promising antibacterial wound dressing made of electrospun poly (glycerol sebacate) (PGS)/gelatin with local delivery of ascorbic acid and pantothenic acid. J Polym Environ 31:2504–2518. https://doi.org/10.1007/s10924-022-02715-8
Ndlovu SP, Ngece K, Alven S, Aderibigbe BA (2021) Gelatin-based hybrid scaffolds: promising wound dressings. Polymers (Basel) 13:2959. https://doi.org/10.3390/polym13172959
Jalilian M, Yeganeh H, Haghighi MN (2008) Synthesis and properties of polyurethane networks derived from new soybean oil-based polyol and a bulky blocked polyisocyanate. Polym Int 57:1385–1394. https://doi.org/10.1002/pi.2485
Wang S, Li Y, Fan X et al (2015) β-cyclodextrin functionalized graphene oxide: an efficient and recyclable adsorbent for the removal of dye pollutants. Front Chem Sci Eng 9:77–83. https://doi.org/10.1007/s11705-014-1450-x
Cornille A, Blain M, Auvergne R et al (2017) A study of cyclic carbonate aminolysis at room temperature: effect of cyclic carbonate structures and solvents on polyhydroxyurethane synthesis. Polym Chem 8:592–604. https://doi.org/10.1039/C6PY01854H
Lambeth RH, Rizvi A (2019) Mechanical and adhesive properties of hybrid epoxy-polyhydroxyurethane network polymers. Polymer (Guildf) 183:121881
Babaahmadi M, Yeganeh H (2023) Poly(vinyl alcohol)-gelatin crosslinked by silane-functionalized guanidyl-hydroxyurethane oligomer as contact-killing non-leaching antibacterial wound dressings. Biomed Mater 18:045017. https://doi.org/10.1088/1748-605X/acd5a0
Pawde SM, Deshmukh K (2008) Characterization of polyvinyl alcohol/gelatin blend hydrogel films for biomedical applications. J Appl Polym Sci 109:3431–3437. https://doi.org/10.1002/app.28454
Tian H, Zeng H, Zha F et al (2020) Synthesis of graphene oxide-supported β-cyclodextrin adsorbent for removal of p-nitrophenol. Water Air Soil Pollut. https://doi.org/10.1007/s11270-020-04865-8
Samadi N, Sabzi M, Babaahmadi M (2018) Self-healing and tough hydrogels with physically cross-linked triple networks based on Agar/PVA/Graphene. Int J Biol Macromol 107:2291–2297. https://doi.org/10.1016/j.ijbiomac.2017.10.104
Babaahmadi M, Sabzi M, Mahdavinia GR, Keramati M (2017) Preparation of amorphous nanocomposites with quick heat triggered shape memory behavior. Polymer (Guildf) 112:26–34. https://doi.org/10.1016/j.polymer.2017.01.074
Sabzi M, Babaahmadi M, Samadi N et al (2017) Graphene network enabled high speed electrical actuation of shape memory nanocomposite based on poly(vinyl acetate). Polym Int 66:665–671. https://doi.org/10.1002/pi.5303
Wan Y-J, Tang L-C, Gong L-X et al (2014) Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon N Y 69:467–480. https://doi.org/10.1016/j.carbon.2013.12.050
Salvatore L, Calò E, Bonfrate V et al (2021) Exploring the effects of the crosslink density on the physicochemical properties of collagen-based scaffolds. Polym Test 93:106966. https://doi.org/10.1016/j.polymertesting.2020.106966
Zhao Y, Sun Z (2018) Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int J Food Prop 20:S2822–S2832. https://doi.org/10.1080/10942912.2017.1381111
Catalina M, Attenburrow GE, Cot J et al (2011) Influence of crosslinkers and crosslinking method on the properties of gelatin films extracted from leather solid waste. J Appl Polym Sci 119:2105–2111. https://doi.org/10.1002/app.32932
Bao C, Guo Y, Song L et al (2011) In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J Mater Chem 21:13290. https://doi.org/10.1039/c1jm11434d
Surnova A, Balkaev D, Musin D et al (2019) Fully exfoliated graphene oxide accelerates epoxy resin curing, and results in dramatic improvement of the polymer mechanical properties. Composites B 162:685–691. https://doi.org/10.1016/j.compositesb.2019.01.020
Tonda-Turo C, Gentile P, Saracino S et al (2011) Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int J Biol Macromol 49:700–706. https://doi.org/10.1016/j.ijbiomac.2011.07.002
Boateng JS, Matthews KH, Stevens HNE, Eccleston GM (2008) Wound healing dressings and drug delivery systems : a review. J Pharm Sci 97:2892–2923. https://doi.org/10.1002/jps
Yari A, Yeganeh H, Bakhshi H (2012) Synthesis and evaluation of novel absorptive and antibacterial polyurethane membranes as wound dressing. J Mater Sci Mater Med 23:2187–2202. https://doi.org/10.1007/s10856-012-4683-6
Poursamar SA, Lehner AN, Azami M et al (2016) The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mater Sci Eng C 63:1–9. https://doi.org/10.1016/j.msec.2016.02.034
Sabzi M, Jiang L, Liu F et al (2013) Graphene nanoplatelets as poly(lactic acid) modifier: linear rheological behavior and electrical conductivity. J Mater Chem A 1:8253. https://doi.org/10.1039/c3ta11021d
Gharibi R, Kazemi S, Yeganeh H, Tafakori V (2019) Utilizing dextran to improve hemocompatibility of antimicrobial wound dressings with embedded quaternary ammonium salts. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.03.185
Teng X, Liu X, Cui Z et al (2020) Rapid and highly effective bacteria-killing by polydopamine/IR780@MnO2–Ti using near-infrared light. Prog Nat Sci Mater Int 30:677–685. https://doi.org/10.1016/j.pnsc.2020.06.003
Weissleder R (2001) A clearer vision for in vivo imaging. Nat Biotechnol 19:316–317. https://doi.org/10.1038/86684
Wang N, Hu B, Chen ML, Wang JH (2015) Polyethylenimine mediated silver nanoparticle-decorated magnetic graphene as a promising photothermal antibacterial agent. Nanotechnology 26:195703. https://doi.org/10.1088/0957-4484/26/19/195703
Li M, Yang X, Ren J et al (2012) Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv Mater 24:1722–1728. https://doi.org/10.1002/adma.201104864
Ma L, Wang Y, Wang Y et al (2020) Polyimide nanocomposites with reduced graphene oxide for enhanced thermal conductivity and tensile strength. Mater Res Express 6:125346. https://doi.org/10.1088/2053-1591/ab5ddf
Lu X, Liang B, Sheng X et al (2020) Enhanced thermal conductivity of polyurethane/wood powder composite phase change materials via incorporating low loading of graphene oxide nanosheets for solar thermal energy storage. Sol Energy Mater Sol Cells 208:110391. https://doi.org/10.1016/j.solmat.2019.110391
Balandin A, Ghosh S, Bao W et al (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907. https://doi.org/10.1021/nl0731872
Li J, Liu X, Zhou Z et al (2019) Lysozyme-assisted photothermal eradication of methicillin-resistant Staphylococcus aureus infection and accelerated tissue repair with natural melanosome nanostructures. ACS Nano 13:11153–11167. https://doi.org/10.1021/acsnano.9b03982
Frattini A, Pellegri N, Nicastro D, de Sanctis O (2005) Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater Chem Phys 94:148–152. https://doi.org/10.1016/j.matchemphys.2005.04.023
Pourjavadi A, Soleyman R (2011) Silver nanoparticles with gelatin nanoshells: photochemical facile green synthesis and their antimicrobial activity. J Nanoparticle Res 13:4647–4658. https://doi.org/10.1007/s11051-011-0428-6
Mock JJ, Barbic M, Smith DR et al (2002) Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys 116:6755. https://doi.org/10.1063/1.1462610
Peter Amaladhas T, Usha M, Naveen S (2013) Sunlight induced rapid synthesis and kinetics of silver nanoparticles using leaf extract of Achyranthes aspera L. and their antimicrobial applications. Adv Mater Lett 4:779–785. https://doi.org/10.5185/amlett.2013.2427
Yin IX, Zhang J, Zhao IS et al (2020) The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed 15:2555–2562. https://doi.org/10.2147/IJN.S246764
Yaragalla S, Bhavitha KB, Athanassiou A (2021) A review on graphene based materials and their antimicrobial properties. Coatings 11:1197. https://doi.org/10.3390/coatings11101197
Choudhary P, Ramalingam B, Das SK (2023) Rational design of antimicrobial peptide conjugated graphene-silver nanoparticle loaded chitosan wound dressing. Int J Biol Macromol 246:125347. https://doi.org/10.1016/j.ijbiomac.2023.125347
Rezapour-Lactoee A, Yeganeh H, Ostad SN et al (2016) Thermoresponsive polyurethane/siloxane membrane for wound dressing and cell sheet transplantation: In-vitro and in-vivo studies. Mater Sci Eng C 69:804–814. https://doi.org/10.1016/j.msec.2016.07.067
Dias JR, Baptista-Silva S, Oliveira CMT et al (2017) In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur Polym J 95:161–173. https://doi.org/10.1016/j.eurpolymj.2017.08.015
Martucci JF, Espinosa JP, Ruseckaite RA (2015) Physicochemical properties of films based on bovine gelatin cross-linked with 1,4-butanediol diglycidyl ether. Food Bioprocess Technol 8:1645–1656. https://doi.org/10.1007/s11947-015-1524-x
Tripodo G, Trapani A, Rosato A et al (2018) Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr Polym 198:124–130. https://doi.org/10.1016/j.carbpol.2018.06.061
Funding
The authors appreciate the partial support of Iran Polymer and Petrochemical Institute.
Author information
Authors and Affiliations
Contributions
M.B. contributed in the acquisition, analysis, and interpretation of data; drafted the work; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. H.Y. contributed to the conception and design of the work; interpretation of data; drafted the work, revised it critically for important intellectual content; approved the version to be submitted; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babaahmadi, M., Yeganeh, H. Antibacterial Polyhydroxyurethane-Gelatin Wound Dressings with In Situ-Generated Silver Nanoparticles or Hyperthermia Induced by Near-Infrared Light Absorption. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03204-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03204-w