Abstract
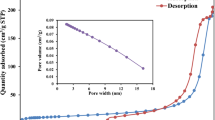
Herein, the biopolymer chitosan (CHI) was functionalized by grafting aromatic groups (benzaldehyde, BZA) onto its backbone using a hydrothermal technique to create an efficient hydrothermally chitosan-benzaldehyde adsorbent (CHI-BZA). The characterization results revealed a mesoporous and crystalline structural features of the CHI-BZA, further supporting that the aromatic ring of BZA was successfully grafted onto CHI. The resulting CHI-BZA product was applied as a promising adsorbent to remove a model of acidic dye (reactive orange 16, RO16) from simulated wastewater. Optimizing the major adsorption factors (A: CHI-BZA dose (0.02–0.08 g); B: pH (4–10); C: time (5–25)) was accomplished by the Box-Behnken design (BBD). The Freundlich isotherm and pseudo-second-order kinetic models match the adsorption profile of RO16 species. The hydrothermally prepared CHI-BZA was found to possess a maximum adsorption capacity (qmax) of 228.9 mg/g for the RO16 dye. The adsorption of RO16 species onto the CHI-BZA surface is controlled by several types of interactions: electrostatic, n-π, π-π, and H-bonding. This work highlights that hydrothermally prepared CHI-BZA may serve as an efficient and promising adsorbent for the removal of acidic dyes from contaminated water.










Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Qahtani SD, Snari RM, Alamrani NA, Aljuhani E, Bayazeed A, Aldawsari AM, El-Metwaly NM (2022) Synthesis and adsorption properties of fibrous-like aerogel from acylhydrazone polyviologen: efficient removal of reactive dyes from wastewater. J Mater Res Technol 18:1822–1833
Yildirim A (2021) Removal of the anionic dye Reactive Orange 16 by chitosan/tripolyphosphate/mushroom. Chem Eng Technol 44(8):1371–1381
Seyedi MS, Sohrabi MR, Motiee F, Mortazavinik S (2020) Synthesis and characterization of activated carbon@ zerovalent iron–nickel nanoadsorbent for highly efficient removal of Reactive Orange 16 from aqueous sample: experimental design, kinetic, isotherm and thermodynamic studies. Res Chem Intermediate 46(3):1645–1662
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat 164:346–360
Duan Y, Zhao J, Qiu X, Deng X, Ren X, Ge W, Yuan H (2022) Coagulation performance and floc properties for synchronous removal of reactive dye and polyethylene terephthalate microplastics. Process Safe Environ Prot 165:66–76
Tang Y, Liu M, He D, Pan R, Dong W, Feng S, Ma L (2022) Efficient electrochemical degradation of X-GN dye wastewater using porous boron-doped diamond electrode. Chemosphere 307:135912
Gaur R, Shahabuddin S, Ahmad I (2022) Novel MAX phase/polyaniline nanocomposite for photocatalytic degradation of toxic industrial dye. Mater Lett 325:132888
Xiang J, Li H, Hei Y, Tian G, Zhang L, Cheng P, Tang N (2022) Preparation of highly permeable electropositive nanofiltration membranes using quaternized polyethyleneimine for dye wastewater treatment. J Water Process Eng 48:102831
Chen CY, Tseng WJ (2022) Preparation of TiN-WN composite particles for selective adsorption of methylene blue dyes in water. Adv Powder Technol 33(2):103423
Priyadarshini B, Patra T, Sahoo TR (2021) An efficient and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies. J Magnesium Alloys 9(2):478–488
Laktif T, Imgharn A, Hsini A, Elhoudi M, Aarab N, Laabd M, Albourine A (2022) Sunflower seed shells@ polyaniline: a novel composite for the removal of pharmaceutical pollutants from wastewater. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2060086
Imgharn A, Aarab N, Hsini A, Naciri Y, Elhoudi M, Haki MA, Albourine A (2022) Application of calcium alginate-PANI@ sawdust wood hydrogel bio-beads for the removal of orange G dye from aqueous solution. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20162-9
Imgharn A, Anchoum L, Hsini A, Naciri Y, Laabd M, Mobarak M, Albourine A (2022) Effectiveness of a novel polyaniline@ Fe-ZSM-5 hybrid composite for Orange G dye removal from aqueous media: Experimental study and advanced statistical physics insights. Chemosphere 295:133786
Hsini A, Naciri Y, Bouziani A, Aarab N, Essekri A, Imgharn A, Albourine A (2021) Polyaniline coated tungsten trioxide as an effective adsorbent for the removal of orange G dye from aqueous media. RSC Adv 11(50):31272–31283
Imgharn A, Hsini A, Naciri Y, Laabd M, Kabli H, Elamine M, Albourine A (2021) Synthesis and characterization of polyaniline-based biocomposites and their application for effective removal of Orange G dye using adsorption in dynamic regime. Chem Phys Lett 778:138811
Abdalla TH, Nasr AS, Bassioni G, Harding DR, Kandile NG (2022) Fabrication of sustainable hydrogels-based chitosan Schiff base and their potential applications. Arab J Chem 15(1):103511
Ge H, Du J (2020) Selective adsorption of Pb (II) and Hg (II) on melamine-grafted chitosan. Int J Biol Macromol 162:1880–1887
Verma M, Lee I, Hong Y, Kumar V, Kim H (2022) Multifunctional β-cyclodextrin-EDTA-chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. Environ Pollut 292:118447
Abdulhameed AS, Jawad AH, Ridwan M, Khadiran T, Wilson LD, Yaseen ZM (2022) Chitosan/carbon-doped TiO2 composite for adsorption of two anionic dyes in solution and gaseous SO2 capture: experimental modeling and optimization. J Polym Environ. https://doi.org/10.1007/s10924-022-02532-z
Coura JC, Profeti D, Profeti LPR (2020) Eco-friendly chitosan/quartzite composite as adsorbent for dye removal. Mater Chem Phys 256:123711
Benosman A, Slimane SK, Roger P (2021) Adsorption of anionic dye by cross-linked chitosan-polyaniline composites. J Water Chem Technol 43(1):14–21
Tanhaei B, Ayati A, Iakovleva E, Sillanpää M (2020) Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: synthesis, characterization and adsorption study. Int J Biol Macromol 164:3621–3631
Zheng W, Kuchukulla RR, Xu X, Zhang D, Zhou L, Zeng Q (2022) Study on synthesis of comb-shaped chitosan-graft-polyethylenimine dithiocarbamate material and its adsorption to heavy metal ions. J Polym Environ 30(2):653–665
Tahira I, Aslam Z, Abbas A, Monim-ul-Mehboob M, Ali S, Asghar A (2019) Adsorptive removal of acidic dye onto grafted chitosan: a plausible grafting and adsorption mechanism. Int J Boil Macromol 136:1209–1218
Hermosillo-Ochoa E, Picos-Corrales LA, Licea-Claverie A (2021) Eco-friendly flocculants from chitosan grafted with PNVCL and PAAc: Hybrid materials with enhanced removal properties for water remediation. Sep Purif Technol 258:118052
Banisheykholeslami F, Hosseini M, Darzi GN (2021) Design of PAMAM grafted chitosan dendrimers biosorbent for removal of anionic dyes: adsorption isotherms, kinetics and thermodynamics studies. Int J Biol Macromol 177:306–316
Ye Y, Zhang T, Lv L, Chen Y, Tang W, Tang S (2021) Functionalization of chitosan by grafting sulfhydryl groups to intensify the adsorption of arsenite from water. Colloids Surf A Physicochem Eng Asp 622:126601
Elwakeel KZ, Elgarahy AM, Al-Bogami AS, Hamza MF, Guibal E (2021) 2-Mercaptobenzimidazole-functionalized chitosan for enhanced removal of methylene blue: batch and column studies. J Environ Chem Eng 9(4):105609
Song X, Chen H, Gong L, Cui J, Wang Y, Wen S, Xiong Y (2022) Synthesis of novel diol modified chitosan and their enhanced selective adsorption behavior for germanium (IV). J Environ Chem Eng 10(1):107082
Ranjbari S, Tanhaei B, Ayati A, Khadempir S, Sillanpää M (2020) Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: mechanism, kinetic, isotherms and thermodynamic study. Int J Biol Macromol 155:421–429
Karimi F, Ayati A, Tanhaei B, Sanati AL, Afshar S, Kardan A, Karaman C (2022) Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; a powerful approach in water treatment. Environ Res 203:111753
Liu X, Zhang Y, Ju H, Yang F, Luo X, Zhang L (2021) Uptake of methylene blue on divinylbenzene cross-linked chitosan/maleic anhydride polymer by adsorption process. Colloids Surf A Physicochem Eng Asp 629:127424
Huang C, Liao H, Ma X, Xiao M, Liu X, Gong S, Zhou X (2021) Adsorption performance of chitosan Schiff base towards anionic dyes: electrostatic interaction effects. Chem Phys Lett 780:138958
Sun Y, Kang Y, Zhong W, Liu Y, Dai Y (2020) A simple phosphorylation modification of hydrothermally cross-linked chitosan for selective and efficient removal of U (VI). J Solid State Chem 292:121731
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Magnet Mater 404:179–189
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619
Yang J, Ao Z, Niu X, Dong J, Wang S, Wu H (2021) Facile one-step synthesis of 3D honeycomb-like porous chitosan bead inlaid with MnFe bimetallic oxide nanoparticles for enhanced degradation of dye pollutant. Int J Biol Macromol 186:829–838
Thatte CS, Rathnam MV, Pise AC (2014) Chitosan-based Schiff base-metal complexes (Mn, Cu, Co) as heterogeneous, new catalysts for the β-isophorone oxidation. J Chem Sci 126:727–737
de Araújo EL, Barbosa HFG, Dockal ER, Cavalheiro ÉTG (2017) Synthesis, characterization and biological activity of Cu (II), Ni (II) and Zn (II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int J Boil Macromol 95:168–176
Babazadeh M, Abolghasemi H, Esmaeili M, Ehsani A, Badiei A (2021) Comprehensive batch and continuous methyl orange removal studies using surfactant modified chitosan-clinoptilolite composite. Sep Purifi Technol 267:118601
Shahraki S, Delarami HS, Khosravi F, Nejat R (2020) Improving the adsorption potential of chitosan for heavy metal ions using aromatic ring-rich derivatives. J Colloid Interface Sci 576:79–89
Shahraki S, Delarami HS, Khosravi F (2019) Synthesis and characterization of an adsorptive Schiff base-chitosan nanocomposite for removal of Pb (II) ion from aqueous media. Int J Biol Macromol 139:577–586
Jawad AH, Abdulhameed AS, Wilson LD, Hanafiah MAKM, Nawawi WI, ALOthman ZA, Rizwan Khan M, (2021) Fabrication of Schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J Polym Environ 29(9):2855–2868
Muthusaravanan S, Balasubramani K, Suresh R, Ganesh RS, Sivarajasekar N, Arul H, Banat F (2021) Adsorptive removal of noxious atrazine using graphene oxide nanosheets: Insights to process optimization, equilibrium, kinetics, and density functional theory calculations. Environ Res 200:111428
Shukla SK, Pandey S, Saha S, Singh HR, Mishra PK, Kumar S, Jha SK (2021) Removal of crystal violet by Cu-chitosan nano-biocomposite particles using Box-Behnken design. J Environ Chem Eng 9(5):105847
Jawad AH, Abdulhameed AS, Surip SN, Sabar S (2020) Adsorptive performance of carbon modified chitosan biopolymer for cationic dye removal: kinetic, isotherm, thermodynamic, and mechanism study. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1807966
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng China 3:320–324
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186:458–465
McKay G (1996) Use of adsorbents for the removal of pollutants from wastewaters, 1st edn. CRC Press, Boca Raton
Hadi M, McKay G, Samarghandi MR, Maleki A, Solaimany Aminabad M (2012) Prediction of optimum adsorption isotherm: comparison of chi-square and Log-likelihood statistics. Desalin Water Treat 49(1–3):81–94
Wang WR, Chen PY, Deng J, Chen Y, Liu HJ (2022) Carbon-dot hydrogels as superior carbonaceous adsorbents for removing perfluorooctane sulfonate from water. Chem Eng J 435:135021
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts, Acta physiochim. URSS 12:327–356
Jemutai-Kimosop S, Orata F, Shikuku VO, Okello VA, Getenga ZM (2020) Insights on adsorption of carbamazepine onto iron oxide modified diatomaceous earth: kinetics, isotherms, thermodynamics, and mechanisms. Environ Res 180:108898
Hoseinzadeh H, Hayati B, Ghaheh FS, Seifpanahi-Shabani K, Mahmoodi NM (2021) Development of room temperature synthesized and functionalized metal-organic framework/graphene oxide composite and pollutant adsorption ability. Mater Res Bullet 142:111408
Kannusamy P, Sivalingam T (2013) Synthesis of porous chitosan–polyaniline/ZnO hybrid composite and application for removal of reactive orange 16 dye. Colloids Surf B Biointerfaces 108:229–238
Malakootian M, Heidari MR (2018) Reactive orange 16 dye adsorption from aqueous solutions by psyllium seed powder as a low-cost biosorbent: kinetic and equilibrium studies. Appl Water Sci 8(7):1–9
Abd Malek NN, Jawad AH, Abdulhameed AS, Ismail K, Hameed BH (2020) New magnetic Schiff’s base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: an optimized process. Int J Biol Macromol 146:530–539
Chandarana H, Subburaj S, Kumar PS, Kumar MA (2021) Evaluation of phase transfer kinetics and thermodynamic equilibria of Reactive Orange 16 sorption onto chemically improved Arachis hypogaea pod powder. Chemosphere 276:130136
Muralikrishnan R, Jodhi C (2021) Biodecolorization of Reactive Orange 16 using biochar produced from groundnut shell (Arachis hypogaea): batch, isotherm, kinetic, and regeneration studies. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01710-8
Chen Z, Zhang ZB, Zeng J, Zhang ZJ, Ma S, Tang CM, Xu JQ (2022) Preparation of polyethyleneimine-modified chitosan/Ce-UIO-66 composite hydrogel for the adsorption of methyl orange. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2022.120079
Acknowledgements
The authors would like to thank the Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam for the research facilities. The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP-2021/1), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by NIN, ASA, AHJ, RR, EY, ZAA, LDW. The first draft of the manuscript was written by NIN, ASA, AHJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Normi, N.I., Abdulhameed, A.S., Razuan, R. et al. One-Step Functionalization of Chitosan Using Aromatic Groups via Hydrothermal Assisted Grafting for Removal of Reactive Orange 16 Dye: Optimization and Adsorption Mechanism. J Polym Environ 31, 1292–1307 (2023). https://doi.org/10.1007/s10924-022-02688-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02688-8