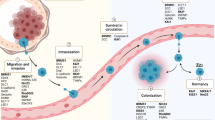
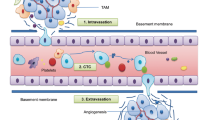
Abstract
Metastasis remains the most deadly aspect of cancer and still evades direct treatment. Clinically and experimentally, primary tumor development and metastasis are distinct processes—locally growing tumors can progress without the development of metastases. The discovery of endogenous molecules that exclusively inhibit metastasis suggests that metastasis is an amenable therapeutic target. By definition, metastasis suppressors inhibit metastasis without inhibiting tumorigenicity and are thus distinct from tumor suppressors. As the biology underlying functional mechanisms of metastasis suppressors becomes clearer, it is evident that metastasis suppressors could be harnessed as direct drug targets, prognostic markers, and to understand the fundamental biology of the metastatic process. Metastasis suppressors vary widely in their cellular localization: they are found in every cellular compartment and some are secreted. In general, metastasis suppressors appear to regulate selectively how cells respond to exogenous signals, by affecting signaling cascades which regulate downstream gene expression. This review briefly summarizes current functional and biochemical data on metastasis suppressors implicated in breast cancer. We also present a schematic integrating known mechanisms for these metastasis suppressors highlighting potential targets for therapeutic intervention.

Similar content being viewed by others
Abbreviations
- AKT:
-
v-akt murine thymoma viral oncogene homolog 1
- AP2:
-
Activating protein 2
- ARID4A:
-
AT rich interactive domain 4A
- CK2:
-
Casein kinase 2
- CRSP3:
-
Co-factor required for Sp1 activity
- CXCR4:
-
Chemokine (C-X-C motif) receptor 4
- EGFR:
-
Epidermal growth factor receptor
- ET1:
-
Endothelin 1
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- GPR54:
-
G-protein coupled receptor 54
- GSK1:
-
Glycogen synthase kinase 1
- GTPase:
-
Guanine tri-phosphatase
- HER2:
-
Human epidermal growth factor receptor 2
- HLA-DR:
-
Human leucocyte antigen-DR
- IL8:
-
Interleukin 8
- JNK:
-
c-Jun NH2 terminal protein kinase
- KSR:
-
Kinase suppressor of Ras
- MAPK:
-
Mitogen activated protein kinase
- MHC1:
-
Major histocompatibility complex class 1
- MLK3:
-
Mixed lineage kinase 3
- MPA:
-
Medroxyprogesterone acetate
- HDAC:
-
Histone deacetylase
- NF-κB:
-
Nuclear factor-kappa B
- NMU:
-
Neuromedin U
- PI3K:
-
Phosphatidyl inositol 3-kinase
- PtdIns(4,5)P2 :
-
Phosphatidyl inositol (4,5) bisphosphate
- PMA:
-
Phorbol myristate acetate
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome 10
- RBBP1:
-
Retinoblastoma binding protein 1
- RhoGAP:
-
Rho GTPase-activating protein
- SDF-1:
-
Stromal derived factor-1
- SUDS3:
-
Suppressor of defective silencing 3
- TGF-β:
-
Transforming growth factor-β
- TXNIP:
-
Thioredoxin interacting protein
- VDUP1:
-
Vitamin D upregulated protein 1
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor 1
References
Page DL, Kidd TE Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Human Pathol 1991;22(12):1232–9.
Rosen PP, Kosloff C, Lieberman PH, Adair F, Braun DW Jr, Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol 1978;2(3):225–51.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57(1):43–66.
Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer 2006;6(12):924–35.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100(1):57–70.
Welch DR. Do we need to redefine a cancer metastasis and staging definitions? Breast Dis 2007;26(1):3–12.
Hynes RO. Metastatic potential: generic predisposition of the primary tumor or rare, metastatic variants—or both? Cell 2003;113(7):821–3.
Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res 1975;35(3):512–6.
Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 1970;45(4):773–82.
Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2(8):563–72.
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438(7069):820–7.
Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889;1:571–3.
Fidler IJ, Gersten DM, Riggs CW. Relationship of host immune status to tumor cell arrest, distribution and survival in experimental metastasis. Cancer 1977;40(1):46–55.
Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res 1980;40(7):2281–7.
Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006;12(8):895–904.
Fidler IJ, Radinsky R. Search for genes that suppress cancer metastasis. J Natl Cancer Inst 1996;88(23):1700–3.
Rinker-Schaeffer CW, O’Keefe JP, Welch DR, Theodorescu D. Metastasis suppressor proteins: discovery, molecular mechanisms and clinical application. Clin Cancer Res 2006;12 (13):3382–9.
Hanai J, Mammoto T, Seth P, Mori K, Karumanchi SA, Barasch J, et al. Lipocalin 2 diminishes invasiveness and metastasis of Ras-transformed cells. J Biol Chem 2005;280(14):13641–7.
Venkatesha S, Hanai J, Seth P, Karumanchi SA, Sukhatme VP. Lipocalin 2 antagonizes the proangiogenic action of ras in transformed cells. Mol Cancer Res 2006;4(11):821–9.
Urtreger AJ, Werbajh SE, Verrecchia F, Mauviel A, Puricelli LI, Kornblihtt AR, et al. Fibronectin is distinctly downregulated in murine mammary adenocarcinoma cells with high metastatic potential. Oncol Rep 2006;16(6):1403–10.
Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol Cell Biol 2006;26(24):9338–51.
Phillips KK, Welch DR, Miele ME, Lee J-H, Wei LL, Weissman BE. Suppression of MDA-MB-435 breast carcinoma cell metastasis following the introduction of human chromosome 11. Cancer Res 1996;56(10):1222–6.
Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res 2000;60(11):2764–9.
Seraj MJ, Harding MA, Gildea JJ, Welch DR, Theodorescu D. The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clin Exp Metastasis 2001;18(6):519–25.
Shevde LA, Samant RS, Goldberg SF, Sikaneta T, Alessandrini A, Donahue HJ, et al. Suppression of human melanoma metastasis by the metastasis suppressor gene, BRMS1. Exp Cell Res 2002;273(2):229–39.
Zhang S, Lin QD, DI W. Suppression of human ovarian carcinoma metastasis by the metastasis-suppressor gene, BRMS1. Int J Gynecol Cancer 2006;16(2):522–31.
Kelly LM, Buggy Y, Hill A, O’Donovan N, Duggan C, McDermott EW, et al. Expression of the breast cancer metastasis suppressor gene, BRMS1, in human breast carcinoma: lack of correlation with metastasis to axillary lymph nodes. Tumor Biol 2005;26(4):213–6.
Lombardi G, Di CC, Capodanno A, Iorio MC, Aretini P, Isola P, et al. High level of messenger RNA for BRMS1 in primary breast carcinomas is associated with poor prognosis. Int J Cancer 2006;120(6):1169–78.
Zhang Z, Yamashita H, Toyama T, Yamamoto Y, Kawasoe T, Iwase H. Reduced expression of the breast cancer metastasis suppressor 1 mRNA is correlated with poor progress in breast cancer. Clin Cancer Res 2006;12(21):6410–4.
Hicks DG, Yoder BJ, Short S, Tarr S, Prescott N, Crowe JP, et al. Loss of BRMS1 protein expression predicts reduced disease-free survival in hormone receptor negative and HER2 positive subsets of breast cancer. Clin Cancer Res 2006;12(22):6702–8.
Hurst DR, Mehta A, Moore BP, Phadke PA, Meehan WJ, Accavitti MA, et al. Breast cancer metastasis suppressor 1 (BRMS1) is stabilized by the Hsp90 chaperone. Biochem Biophys Res Commun 2006;348(4):1429–35.
Meehan WJ, Samant RS, Hopper JE, Carrozza MJ, Shevde LA, Workman JL, et al. Breast cancer metastasis suppressor 1 (BRMS1) forms complexes with retinoblastoma-binding protein 1 (RBP1) and the mSin3 histone deacetylase complex and represses transcription. J Biol Chem 2004;279(2):1562–9.
Cicek M, Fukuyama R, Welch DR, Sizemore N, Casey G. Breast cancer metastasis suppressor 1 inhibits gene expression by targeting nuclear factor-kappaB activity. Cancer Res 2005;65(9):3586–95.
Liu Y, Smith PW, Jones DR. Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol Cell Biol 2006;26(23):8683–96.
Samant RS, Clark DW, Fillmore RA, Cicek M, Metge BJ, Chandramouli KH, et al. Breast cancer metastasis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NF-Kappa B activation. Mol Cancer 2007;6(1):6–14.
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 1999;401(6748):82–5.
DeWald DB, Torabinejad J, Samant RS, Johnston D, Erin N, Shope JC, et al. Metastasis suppression by breast cancer metastasis suppressor 1 involves reduction of phosphoinositide signaling in MDA-MB-435 breast carcinoma cells. Cancer Res 2005;65(3):713–7.
Samant RS, Seraj MJ, Saunders MM, Sakamaki T, Shevde LA, Harms JF, et al. Analysis of mechanisms underlying BRMS1 suppression of metastasis. Clin Exp Metastasis 2001;18(8):683–93.
Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995;268(5212):884–6.
Ichikawa T, Ichikawa Y, Dong J, Hawkins AL, Griffin CA, Isaacs WB, et al. Localization of metastasis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res 1992;52(12):3486–90.
Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005;6(10):801–11.
Sugiura T, Berditchevski F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2). J Cell Biol 1999;146(6):1375–89.
Longo N, Yanez-Mo M, Mittelbrunn M, de la RG, Munoz ML, Sanchez-Madrid F, et al. Regulatory role of tetraspanin CD9 in tumor-endothelial cell interaction during transendothelial invasion of melanoma cells. Blood 2001;98(13):3717–26.
Zhou B, Liu L, Reddivari M, Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res 2004;64(20):7455–63.
Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene 2006;25(16):2367–78.
Takaoka A, Hinoda Y, Sato S, Itoh F, Adachi M, Hareyama M, et al. Reduced invasive and metastatic potentials of KAI1-transfected melanoma cells. Jpn J Cancer Res 1998;89(4):397–404.
Yang XH, Welch DR, Phillips KK, Weissman BE, Wei LL. KAI1, a putative marker for metastatic potential in human breast cancer. Cancer Lett 1997;119(2):149–55.
Phillips KK, White AE, Hicks DJ, Welch DR, Barrett JC, Wei LL, et al. Correlation between reduction of metastasis in the MDA-MB-435 model system and increased expression of the Kai-1 protein. Mol Carcinog 1998;21(3):111–20.
Yang X, Wei L, Tang C, Slack R, Montgomery E, Lippman M. KAI1 protein is down-regulated during the progression of human breast cancer. Clin Cancer Res 2000;6(9):3424–9.
Lombardi DP, Geradts J, Foley JF, Chiao C, Lamb PW, Barrett JC. Loss of KAI1 expression in the progression of colorectal cancer. Cancer Res 1999;59(22):5724–31.
Liu FS, Dong JT, Chen JT, Hsieh YT, Ho ES, Hung MJ. Frequent down-regulation and lack of mutation of the KAI1 metastasis suppressor gene in epithelial ovarian carcinoma. Gynecol Oncol 2000;78(1):10–5.
Liu FS, Chen JT, Dong JT, Hsieh YT, Lin AJ, Ho ESC, et al. KAI1 metastasis suppressor gene is frequently down-regulated in cervical carcinoma. Am J Pathol 2001;159(5):1629–34.
Liu FS, Dong JT, Chen JT, Hsieh YT, Ho ES, Hung MJ, et al. KAI1 metastasis suppressor protein is down-regulated during the progression of human endometrial cancer. Clin Cancer Res 2003;9(4):1393–8.
Farhadieh RD, Smee R, Ow K, Yang JL, Russell PJ, Crouch R, et al. Down-regulation of KAI1/CD82 protein expression in oral cancer correlates with reduced disease free survival and overall patient survival. Cancer Lett 2004;213(1):91–8.
Goncharuk VN, del-Rosario A, Kren L, Anwar S, Sheehan CE, Carlson JA, et al. Co-downregulation of PTEN, KAI-1, and nm23-H1 tumor/metastasis suppressor proteins in non-small cell lung cancer. Ann Diagn Pathol 2004;8(1):6–16.
Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, et al. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res 1996;56(19):4387–90.
Mashimo T, Bandyopadhyay S, Goodarzi G, Watabe M, Pai SK, Gross SC, et al. Activation of the tumor metastasis suppressor gene, KAI1, by etoposide is mediated by p53 and c-Jun genes. Biochem Biophys Res Commun 2000;274(2):370–6.
Shibagaki N, Hanada K, Yamashita H, Shimada S, Hamada H. Overexpression of CD82 on human T cells enhances LFA-1/ICAM-1-mediated cell–cell adhesion: functional association between CD82 and LFA-1 in T cell activation. Eur J Immunol 1999;29(12):4081–91.
Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29). J Immunol 1996;157(5):2039–2047.
Horvath G, Serru V, Clay D, Billard M, Boucheix C, Rubinstein E. CD19 is linked to the integrin-associated tetraspans CD9, CD81, and CD82. J Biol Chem 1998;273(46):30537–43.
Lee JH, Park SR, Chay KO, Seo YW, Kook H, Ahn KY, et al. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res 2004;64(12):4235–43.
Delaguillaumie A, Harriague J, Kohanna S, Bismuth G, Rubinstein E, Seigneuret M, et al. Tetraspanin CD82 controls the association of cholesterol-dependent microdomains with the actin cytoskeleton in T lymphocytes: relevance to co-stimulation. J Cell Sci 2004;117(Pt 22):5269–82.
Angelisova P, Hilgert I, Horejsi V. Association of four antigens of the tetraspans family (CD37, CD53, TAPA-1, and R2/C33) with MHC class II glycoproteins. Immunogenetics 1994;39(4):249-56.
Szollosi J, Horejsi V, Bene L, Angelisova P, Damjanovich S. Supramolecular complexes of MHC class I, MHC class II, CD20, and tetraspan molecules (CD53, CD81, and CD82) at the surface of a B cell line JY. J Immunol 1996;157(7):2939–46.
Zhang XA, Lane WS, Charrin S, Rubinstein E, Liu L. EWI2/PGRL associates with the metastasis suppressor KAI1/CD82 and inhibits the migration of prostate cancer cells. Cancer Res 2003;63(10):2665–74.
Delaguillaumie A, Lagaudriere-Gesbert C, Popoff MR, Conjeaud H. Rho GTPases link cytoskeletal rearrangements and activation processes induced via the tetraspanin CD82 in T lymphocytes. J Cell Sci 2002;115(Pt 2):433–43.
Odintsova E, Sugiura T, Berditchevski F. Attenuation of EGF receptor signaling by a metastasis suppressor, the tetraspanin CD82/KAI-1. Curr Biol 2000;10(16):1009–12.
Odintsova E, Voortman J, Gilbert E, Berditchevski F. Tetraspanin CD82 regulates compartmentalisation and ligand-induced dimerization of EGFR. J Cell Sci 2003;116(Pt 22):4557–66.
Bandyopadhyay S, Zhan R, Chaudhuri A, Watabe M, Pai SK, Hirota S, et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med 2006;12(8):933–8.
Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, et al. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 1993;261(5125):1182–4.
Chaudhuri A, Nielsen S, Elkjaer ML, Zbrzezna V, Fang F, Pogo AO. Detection of Duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs. Blood 1997;89(2):701–12.
Marreiros A, Dudgeon K, Dao V, Grimm MO, Czolij R, Crossley M, et al. KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cells. Oncogene 2005;24(4):637–49.
Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and β-catenin complexes. Nature 2005;434(7035):921–6.
Jackson P, Ow K, Yardley G, Delprado W, Quinn DI, Yang JL, et al. Downregulation of KAI1 mRNA in localised prostate cancer and its bony metastases does not correlate with p53 overexpression. Prostate Cancer Prostatic Dis 2003;6(2):174–81.
Akita H, Iizuka A, Hashimoto Y, Kohri K, Ikeda K, Nakanishi M. Induction of KAI-1 expression in metastatic cancer cells by phorbol esters. Cancer Lett 2000;153(1–2):79–83.
Sekita N, Suzuki H, Ichikawa T, Kito H, Akakura K, Igarashi T, et al. Epigenetic regulation of the KAI1 metastasis suppressor gene in human prostate cancer cell lines. Jpn J Cancer Res 2001;92(9):947–51.
Welch DR, Chen P, Miele ME, McGary CT, Bower JM, Weissman BE, et al. Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene 1994;9(1):255–62.
Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996;88(23):1731–7.
Goldberg SF, Miele ME, Hatta N, Takata M, Paquette-Straub CA, Freedman LP, et al. Melanoma metastasis suppression by chromosome 6: Evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res 2003;63(2):432–40.
Mitchell DC, Stafford LJ, Li D, Bar-Eli M, Liu M. Transcriptional regulation of KiSS-1 gene regulation in metastatic melanoma by specificity protein-1 and its coactivator DRIP-130. Oncogene 2007;26(12):1739-47.
Mitchell DC, Stafford LJ, Li D, Bar-Eli M, Liu M. Transcriptional regulation of KiSS-1 gene expression in metastatic melanoma by specificity protein-1 and its coactivator DRIP-130. Oncogene 2006;DOI 10.1038/sj.onc.1209963.
Lee J-H, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res 1997;57(12):2384–7.
Jiang Y, Berk M, Singh LS, Tan HY, Yin LH, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis 2005;22(5):369–76.
Nash KT, Welch DR. The KISS1 metastasis suppressor: mechanistic insights and clinical utility. Front Biosci 2006;11(1):647–59.
Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab 2003;88(2):914–9.
Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001;276(37):34631–6.
Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001;411(6837):613–7.
Nash KT, Phadke PA, Navenot J-M, Hurst DR, Accavitti-Loper MA, Sztul E, et al. KISS1 metastasis suppressor secretion, multiple organ metastasis suppression, and maintenance of tumor dormancy. J Natl Cancer Inst 2007;99(4):309–21.
Navenot JM, Wang Z, Chopin M, Fujii N, Peiper SC. Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: a potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Res 2005;65(22):10450–6.
Goldberg SF, Harms JF, Quon K, Welch DR. Metastasis-suppressed C8161 melanoma cells arrest in lung but fail to proliferate. Clin Exp Metastasis 1999;17(7):601–7.
Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology 2007;148(1):140–7.
Yan CH, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NFκB binding to the promoter as a consequence of IκBα-induced block of p65/p50 nuclear translocation. J Biol Chem 2001;276(2):1164–72.
Goldberg SF, Lee JH, Welch DR. KiSS-1: a suppressor of metastasis. Jikken Igaku (Experimental Medicine—Japanese) 1998;16(16):37–46.
Yoshida BA, Dubauskas Z, Chekmareva MA, Christiano TR, Stadler WM, Rinker-Schaeffer CW. Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res 1999;59(21):5483–7.
Su GH, Song JJ, Repasky EA, Schutte M, Kern SE. Mutation rate of MAP2K4/MKK4 in breast carcinoma. Human Mutat 2002;19(1):81–5.
Yamada SD, Hickson JA, Hrobowski Y, Vander Griend DJ, Benson D, Montag A, et al. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res 2002;62(22):6717–23.
Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 Kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res 2006;66(4):2264–70.
Stark AM, Tongers K, Maass N, Mehdorn HM, Held-Feindt J. Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. J Cancer Res Clin Oncol 2004;131(3):191–8.
Couvelard A, Hu J, Steers G, O’Toole D, Sauvanet A, Belghiti J, et al. Identification of potential therapeutic targets by gene-expression profiling in pancreatic endocrine tumors. Gastroenterology 2006;131(5):1597–610.
Wang L, Pan Y, LeDai J. Evidence of MKK4 pro-oncogenic activity in breast and pancreatic tumors. Oncogene 2004;23(35):5978–85.
Robinson VL, Hickson JA, Vander Griend DJ, Dubauskas Z, Rinker-Schaeffer CW. MKK4 and metastasis suppression: a marriage of signal transduction and metastasis research. Clin Exp Metastasis 2003;20(1):25–30.
Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol 1998;10(2):205–19.
Shen YH, Godlewski J, Zhu J, Sathyanarayana P, Leaner V, Birrer MJ, et al. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J Biol Chem 2003;278(29):26715–21.
Vander Griend DJ, Kocherginsky M, Hickson JA, Stadler WM, Lin AN, Rinker-Schaeffer CW. Suppression of metastatic colonization by the context-dependent activation of the c-jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res 2005;65(23):10984–91.
Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988;80(3):200–4.
Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembranes 2000;32(3):247–58.
Hartsough MT, Steeg PS. Nm23/nucleoside diphosphate kinase in human cancers. J Bioenerg Biomembranes 2000;32(3):301–8.
Leone A, Seeger RC, Hong CM, Hu YY, Arboleda J, Brodeur GM, et al. Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastomas. Oncogene 1993;8(4):855–65.
Leone A, Flatow U, King CR, Sandeen MA, Margulies IMK, Liotta LA, et al. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell 1991;65(1):25–35.
Suzuki E, Ota T, Tsukuda K, Okita A, Matsuoka K, Murakami M, et al. nm23-H1 reduces in vitro cell migration and the liver metastatic potential of colon cancer cells by regulating myosin light chain phosphorylation. Int J Cancer 2004;108(2):207–11.
Kantor JD, McCormick B, Steeg PS, Zetter BR. Inhibition of cell motility after nm23 transfection of human and murine tumor cells. Cancer Res 1993;53(9):1971–3.
Scambia G, Ferrandina G, Marone M, Benedetti PP, Giannitelli C, Piantelli M, et al. nm23 in ovarian cancer: correlation with clinical outcome and other clinicopathologic and biochemical prognostic parameters. J Clin Oncol 1996;14(2):334–42.
Yamashita H, Kobayashi S, Iwase H, Itoh Y, Kuzishima T, Iwata H, et al. Analysis of oncogenes and tumor suppressor genes in human breast cancer. Jpn J Cancer Res 1993;84(8):871–8.
Tommasi S, Fedele V, Crapolicchio A, Bellizzi A, Paradiso A, Reshkin SJ. ErbB2 and the antimetastatic nm23/NDP kinase in regulating serum induced breast cancer invasion. Int J Mol Med 2003;12(1):131–4.
Nakopoulou LL, Tsitsimelis D, Lazaris AC, Tzonou A, Gakiopoulou H, Dicoglou CC, et al. Nm-23, c-erbB-2, and progesterone receptor expression in invasive breast cancer: correlation with clinicopathologic parameters. Cancer Detect Prev 1999;23(4):297–308.
Ohba K, Miyata Y, Koga S, Kanda S, Kanetake H. Expression of nm23-H1 gene product in sarcomatous cancer cells of renal cell carcinoma: correlation with tumor stage and expression of matrix metalloproteinase-2, matrix metalloproteinase-9, sialyl Lewis X, and c-erbB-2. Urology 2005;65(5):1029–34.
Kuppers DA, Lan K, Knight JS, Robertson ES. Regulation of matrix metalloproteinase 9 expression by Epstein-Barr virus nuclear antigen 3C and the suppressor of metastasis Nm23-H1. J Virol 2005;79(15):9714–24.
Zhu JH, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, et al. Interaction of the Ras-related protein associated with diabetes Rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci 1999;96(26):14911–8.
Tseng YH, Vicent D, Zhu JH, Niu YL, Adeyinka A, Moyers JS, et al. Regulation of growth and tumorigenicity of breast cancer cells by the low molecular weight GTPase Rad and Nm23. Cancer Res 2001;61(5):2071–9.
Engel M, Seifert M, Theisinger B, Seyfert U, Welter C. Glyceraldehyde-3-phosphate dehydrogenase and Nm23-H1/Nucleoside diphosphate kinase A—Two old enzymes combine for the novel Nm23 protein phosphotransferase function. J Biol Chem 1998;273(32):20058–65.
Pinon VP, Millot G, Munier A, Vassy J, Linares-Cruz G, Capeau J, et al. Cytoskeletal association of the A and B nucleoside diphosphate kinases of interphasic but not mitotic human carcinoma cell lines: specific nuclear localization of the B subunit. Exp Cell Res 1999;246(2):355–67.
Kimura N, Shimada N. Evidence for complex formation between GTP binding protein(Gs) and membrane-associated nucleoside diphosphate kinase. Biochem Biophys Res Commun 1990;168(1):99–106.
Biondi RM, Engel M, Sauane M, Welter C, Issinger OG, Jimenez de AL, et al. Inhibition of nucleoside diphosphate kinase activity by in vitro phosphorylation by protein kinase CK2. Differential phosphorylation of NDP kinases in HeLa cells in culture. FEBS Lett 1996;399(1–2):183–7.
Salerno M, Ouatas T, Palmieri D, Steeg PS. Inhibition of signal transduction by the nm23 metastasis suppressor: possible mechanisms. Clin Exp Metastasis 2003;20(1):3–10.
Biggs J, Hersperger E, Steeg PS, Liotta LA, Shearn A. A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell 1990;63(5):933–40.
Freije JMP, Blay P, MacDonald NJ, Manrow RE, Steeg PS. Site-directed mutation of Nm23-H1. Mutations lacking motility suppressive capacity upon transfection are deficient in histidine-dependent protein phosphotransferase pathways in vitro. J Biol Chem 1997;272(9):5525–32.
Ma DQ, McCorkle JR, Kaetzel DM. The metastasis suppressor NM23-H1 possesses 3′–5′ exonuclease activity. J Biol Chem 2004;279(17):18073–84.
MacDonald NJ, de la Rosa A, Benedict MA, Freije JM, Krutsch H, Steeg PS. A serine phosphorylation of Nm23, and not its nucleoside diphosphate kinase activity, correlates with suppression of tumor metastatic potential. J Biol Chem 1993;268(34):25780–9.
Steeg PS, de la Rosa A, Flatow U, MacDonald NJ, Benedict M, Leone A. Nm23 and breast cancer metastasis. Breast Cancer Res Treat 1993;25(2):175–87.
Sacks DB. The role of scaffold proteins in MEK/ERK signalling. Biochem Soc Trans 2006;34(Pt 5):833–6.
Muller J, Ory S, Copeland T, Piwnica-Worms H, Morrison DK. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell 2001;8(5):983–93.
Hartsough MT, Morrison DK, Salerno M, Palmieri D, Ouatas T, Mair M, et al. Nm23-H1 metastasis suppressor phosphorylation of kinase suppressor of ras via a histidine protein kinase pathway. J Biol Chem 2002;277(35):32389–99.
Mink S, Hartig E, Jennewein P, Doppler W, Cato AC. A mammary cell-specific enhancer in mouse mammary tumor virus DNA is composed of multiple regulatory elements including binding sites for CTF/NFI and a novel transcription factor, mammary cell-activating factor. Mol Cell Biol 1992;12(11):4906–18.
Welte T, Garimorth K, Philipp S, Jennewein P, Huck C, Cato AC, et al. Involvement of Ets-related proteins in hormone-independent mammary cell-specific gene expression. Eur J Biochem 1994;223(3):997–1006.
Malewski T, Zwierzchowski L. Changes of tissue-specific transcription factors in the rabbit mammary gland during pregnancy and lactation. Tsitol Genet 1997;31(4):58–69.
Ouatas T, Clare SE, Hartsough MT, De L, Steeg PS. MMTV-associated transcription factor binding sites increase nm23-H1 metastasis suppressor gene expression in human breast carcinoma cell lines. Clin Exp Metastasis 2002;19(1):35–42.
Palmieri D, Halverson DO, Ouatas T, Horak CE, Salerno M, Johnson J, et al. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst 2005;97(9):632–42.
Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, et al. Effects of Raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst 2003;95(12):878–89.
Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B, et al. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res 2005;11(20):7392–7.
Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 1999;401(6749):173–7.
Park S, Yeung ML, Beach S, Shields JM, Yeung KC. RKIP downregulates B-Raf kinase activity in melanoma cancer cells. Oncogene 2005;24(21):3535–40.
Trakul N, Menard RE, Schade GR, Qian ZH, Rosner MR. Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. J Biol Chem 2005;280(26):24931–40.
Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R, et al. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem 2004;279(17):17515–23.
Molinaro RJ, Jha BK, Malathi K, Varambally S, Chinnaiyan AM, Silverman RH. Selection and cloning of poly(rC)-binding protein 2 and Raf kinase inhibitor protein RNA activators of 2′,5′-oligoadenylate synthetase from prostate cancer cells. Nucleic Acids Res 2006;34(22):6684–95.
Rusch L, Zhou A, Silverman RH. Caspase-dependent apoptosis by 2′,5′-oligoadenylate activation of RNase L is enhanced by IFN-beta. J Interferon Cytokine Res 2000;20(12):1091–100.
Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet 2007;369(9574):1742–57.
Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ, et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 2006;439(7072):95–9.
Adra CN, Kobayashi H, Rowley JD, Lim B. Assignment of the human GDID4 gene, a GDP/GTP-exchange regulator, to chromosome 12p12.3. Genomics 1994;24(1):188–90.
Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, et al. RhoGD12 is an invasion and metastasis suppressor gene in human cancer. Cancer Res 2002;62(22):6418–23.
Titus B, Frierson HF, Conaway M, Ching K, Guise T, Chirgwin J, et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGD12. Cancer Res 2005;65(16):7320–7.
Wu Y, McRoberts K, Berr SS, Frierson HF Jr, Conaway M, Theodorescu D. Neuromedin U is regulated by the metastasis suppressor RhoGDI2 and is a novel promoter of tumor formation, lung metastasis and cancer cachexia. Oncogene 2006;26(5):765–73.
Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer 2003;97(3):779–84.
Mohammad KS, Guise TA. Mechanisms of osteoblastic metastases: role of endothelin-1. Clin Orthop Relat Res 2003;415:S67–S74.
Su B, Zheng Q, Vaughan MM, Bu Y, Gelman IH. SSeCKS metastasis-suppressing activity in MatLyLu prostate cancer cells correlates with vascular endothelial growth factor inhibition. Cancer Res 2006;66(11):5599–607.
Taback B, Giuliano AE, Lai R, Hansen N, Singer FR, Pantel K, et al. Epigenetic analysis of body fluids and tumor tissues: application of a comprehensive molecular assessment for early-stage breast cancer patients. Ann N Y Acad Sci 2006;1075211–21.
Schwarzenbach H, Chun FK, Lange I, Carpenter S, Gottberg M, Erbersdobler A, et al. Detection of tumor-specific DNA in blood and bone marrow plasma from patients with prostate cancer. Int J Cancer 2007;120(7):1457–63.
Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of nuclear factor-kappab, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res 2007;13(1):59–67.
Park BK, Zhang HL, Zeng QH, Dai JL, Keller ET, Giordano T, et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 2007;13(1):62–9.
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000;148(4):779–90.
Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 2001;61(9):3819–25.
Gao AC, Lou W, Dong JT, Isaacs JT. CD44 is a metastasis suppressor gene for prostatic cancer located on human chromosome 11p13. Cancer Res 1997;57(5):846–9.
Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res 1998;58(10):2196–9.
Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res 2003;63(8):1731–6.
Fujita H, Okada F, Hamada J, Hosokawa M, Moriuchi T, Koya RC, et al. Gelsolin functions as a metastasis suppressor in B16-BL6 mouse melanoma cells and requirement of the carboxyl-terminus for its effect. Int J Cancer 2001;93(6):773–80.
Nuntharatanapong N, Chen K, Sinhaseni P, Keaney JF Jr. EGF receptor-dependent JNK activation is involved in arsenite-induced p21Cip1/Waf1 upregulation and endothelial apoptosis. Am J Physiol Heart Circ Physiol 2005;289(1):H99–H107.
Gautam A, Bepler G. Suppression of lung tumor formation by the regulatory subunit of ribonucleotide reductase. Cancer Res 2006;66(13):6497–502.
Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, et al. SSeCKS regulates angiogenesis and tight junction formation in blood–brain barrier. Nat Med 2003;9(7):900–6.
Martinez R, Setien F, Voelter C, Casado S, Quesada MP, Schackert G, et al. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis 2007;DOI 10.1093/carcin/bgm014.
Jeschke U, Mylonas I, Shabani N, Kunert-Keil C, Schindlbeck C, Gerber B, et al. Expression of sialyl lewis X, sialyl Lewis A, E-cadherin and cathepsin-D in human breast cancer: immunohistochemical analysis in mammary carcinoma in situ, invasive carcinomas and their lymph node metastasis. Anticancer Res 2005;25(3A):1615–22.
Kallakury BV, Yang F, Figge J, Smith KE, Kausik SJ, Tacy NJ, et al. Decreased levels of CD44 protein and mRNA in prostate carcinoma. Correlation with tumor grade and ploidy. Cancer 1996;78(7):1461–9.
Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Tsukada T, Miura K, et al. PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in prostate and breast cancer. Cancer Res 2004;64(21):7655–60.
Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer—Loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol 2003;162(2):609–17.
Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res 2004;10(4):1379–83.
Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP Kinase in thyroid cancer cells. J Clin Endocrinol Metab 2002;87(5):2399.
Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson HF Jr. Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res 2004;10(11):3800–6.
Xia W, Unger P, Miller L, Nelson J, Gelman IH. The Src-suppressed C kinase substrate, SSeCKS, is a potential metastasis inhibitor in prostate cancer. Cancer Res 2001;61(14):5644–51.
Acknowledgments
KSV is supported by a post-doctoral fellowship (PDF1122006) from the Susan G. Komen For The Cure. Work from the Welch laboratory has been generously supported by US Public Health Service grants CA62168, CA87728, CA89019, U.S. Army Medical Research and Materiel Command grants DAMD-17-01-0358, DAMD-17-02-1-0541, and DAMD17-03-01-0584 and the National Foundation for Cancer Research—Center for Metastasis Research. We wish to thank members of the Welch lab for their critical review of this manuscript and excellent suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaidya, K.S., Welch, D.R. Metastasis Suppressors and Their Roles in Breast Carcinoma. J Mammary Gland Biol Neoplasia 12, 175–190 (2007). https://doi.org/10.1007/s10911-007-9049-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-007-9049-1