Abstract
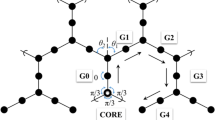
A geometrical approach to two distances in Fréchet-type dendrimers is presented in this paper: the dependence on the conformation of the distance between the centres of two consecutive phenyls (i.e. the two phenyl rings of a benzyl-phenyl-ether motif), and the calculation of the radius of a G1 dendron (i.e. the distance between the C1 atom of the focal phenyl ring and the C4 atom of a phenyl ring superior to it), as a function of torsion angles. This approach enables determination of the extreme values of these distances.
Similar content being viewed by others
References
S. Gestermann, R. Hesse, B. Windisch, F. Vögtle, in Stimulating Concepts in Chemistry, ed. by F. Vögtle, J.F. Stoddart, M. Shibasaki (Wiley, Weinheim, 2000) pp. 187–198
Newkome G.R., Moorefield C., Vögtle F.: Dendrimers and Dendrons. Wiley, New York (2001)
Fréchet J.M.J., Tomalia D.A: Dendrimers and Other Dendritic Polymers. Wiley, Weinheim (2001)
Hecht S.: J. Polym. Sci. Part A 41, 1047–1058 (2003)
Reek J.N.H., Arévalo S., van Heerbeek R., Kamer P.C.J., van Leeuwen P.W.N.M.: Adv. Catal. 49, 71–151 (2006)
Vögtle F., Richardt G., Werner N.: Dendrimer Chemistry. Wiley, New York (2009)
Carlmark A., Hawker C., Hulta A., Malkoch M.: Chem. Soc. Rev. 38, 352–362 (2009)
Rolland O., Turrin C.-O., Caminade A.-M., Majoral J.-P.: New J. Chem. 33, 1809–1824 (2009)
Tekade R.K., Kumar P.V., Jain N.K.: Chem. Rev. 109, 49–87 (2009)
Dufès C., Uchegbu I.F., Schätzlein A.G.: Adv. Drug Deliv. Rev. 57, 2177–2202 (2005)
Coles D.J., Toth I.: Curr. Drug Deliv. 6, 338–342 (2009)
Theodossiou T.A., Pantos A., Tsogas I., Paleos C.M.: Chem. Med. Chem. 3, 1635–1643 (2008)
Guillot-Nieckowski M., Eisler S., Diederich F.: New J. Chem. 31, 1111–1127 (2007)
Boas U., Heergaard P.M.H.: Chem. Soc. Rev. 33, 43–63 (2004)
Medina S.H., El-Sayed M.E.H.: Chem. Rev. 109, 3141–3157 (2009)
Cheng Y., Xu T.: Eur. J. Medi. Chem. 43, 2291–2297 (2008)
Cheng Y., Xu Z., Ma M., Xu T.: J. Pharm. Sci. 97, 123–143 (2008)
Astruc D., Chardac F.: Chem. Rev. 101, 2991–3024 (2001)
Oosterom G.E., Reek J.N.H., Kamer P.C.J., van Leeuwen P.W.N.M.: Angew. Chem. Int. Ed. 40, 1828–1849 (2001)
Chung Y.-M., Rhee H.-K.: Korean J. Chem. Eng. 21, 81–97 (2004)
Méry D., Astruc D.: Coord. Chem. Rev. 250, 1965–1979 (2006)
Gade, L.H. (eds): Dendrimer Catalysis. Springer, Berlin (2006)
Newkome G.R., Yoo K.S., Hwang S.-H., Moorefield C.N.: Tetrahedron 59, 3955–3964 (2003)
Higuchi M., Hayashi A., Kurth D.G.: J. Nanosci. Nanotechnol. 6, 1533–1551 (2006)
Tor Y.: C. R. Chimie 6, 755–766 (2003)
E. Constable, Chem. Commun. 1073–1080 (1997)
Hwang S.-H., Shreiner C.D., Moorefield C.N., Newkome G.R.: New J. Chem. 31, 1192–1217 (2007)
Balzani V., Bergamini G., Ceroni P., Vögtle F.: Coord. Chem. Rev. 251, 525–535 (2007)
Hwang S.-H., Moorefield C.N., Newkome G.R.: Chem. Soc. Rev. 37, 2543–2557 (2008)
Lo S.-C., Burn P.L.: Chem. Rev. 107, 1097–1116 (2007)
Naylor A.M., Goddard W.A. III, Kiefer G.E., Tomalia D.A.: J. Am. Chem. Soc. 111, 2339–2341 (1989)
Scherrenberg R., Coussens B., van Vliet P., Edouard G., Brackman J., de Brabander E., Mortensen K.: Macromolecules 31, 456–461 (1998)
Zhou T., Bor C.S.: Macromolecules 38, 8554–8561 (2005)
Welch P., Muthukumar M.: Macromolecules 31, 5892–5897 (1998)
Timoshenko E.G., Kuznetsov Y.A., Connolly R.: J. Chem. Phys. 117, 9050–9062 (2002)
Ballauff M., Likos C.N.: Angew. Chem. Int. Ed. 43, 2998–3020 (2004)
Hong T., Kim H.-I.: Macromol. Res. 12, 178–188 (2004)
Ortiz W., Roitberg A.E., Krause J.L.: J. Phys. Chem. B 108, 8218–8225 (2004)
Karatasos K., Adolf D.B., Davies G.R.: J. Chem. Phys. 115, 5310–5318 (2001)
Wooley K.L., Klug C.A., Tasaki K., Schaefer J.: J. Am. Chem. Soc. 119, 53–58 (1997)
Gorman C.B., Hager M.W., Parkhurst B.L., Smith J.C.: Macromolecules 31, 815–822 (1998)
Hawker C.J., Wooley K.L., Fréchet J.M.J.: J. Am. Chem. Soc. 115, 4375–4376 (1993)
De Backer S., Prinzie Y., Verheijen W., Smet M., Desmedt K., Dehaen W., De Schryver F.C.: J. Phys. Chem. A 102, 5451–5455 (1998)
Mourey T.H., Turner S.R., Rubinstein M., Fréchet J.M.J., Hawker C.J., Wooley K.L.: Macromolecules 25, 2401–2406 (1992)
Hawker C.J., Fréchet J.M.J.: J. Am. Chem. Soc. 112, 7638–7647 (1990)
Hawker C.J., Fréchet J.M.J.: Macromolecules 23, 4726–4729 (1990)
Hawker C.J., Fréchet J.M.J.: J. Chem. Soc., Chem. Commun. 1990, 1010–1013 (1990)
Grayson S.M., Fréchet J.M.J.: Chem. Rev. 101, 3819–3868 (2001)
Zimmerman S.C., Wendland M.S., Rakow N.A., Zharov I., Suslick K.S.: Nature 418, 399–403 (2002)
Chow H.-F., Chan I.Y.-K., Mak C.C.: Tetrahedron Lett. 36, 8633–8636 (1995)
Chow H.-F., Chan I.Y.-K., Mak C.C., Ng M.-K.: Tetrahedron 52, 4277–4290 (1996)
Mak C.C., Chow H.-F.: Macromolecules 30, 1228–1230 (1997)
Nishiyama N., Stapert H.R., Zhang G.-D., Takasu D., Jiang D.-L., Nagano T., Aida T., Kataoka K.: Bioconj. Chem. 14, 58–66 (2003)
Malkoch M., Schleicher K., Drockenmuller E., Hawker C.J., Russell T.P., Wu P., Fokin V.V.: Macromolecules 38, 3663–3678 (2005)
Stadler A.-M., Brelot L.: Cryst. Growth Des. 10, 2285–2290 (2010)
Hübner G.M., Nachtsheim G., Li Q.Y., Seel C., Vögtle F.: Angew. Chem. Int. Ed. 39, 1269–1272 (2000)
Percec V., Won B.C., Peterca M., Heiney P.A.: J. Am. Chem. Soc. 129, 11265–11278 (2007)
Percec V., Cho W.-D., Ungar G.: J. Am. Chem. Soc. 122, 10273–10281 (2000)
Percec V., Imam M.R., Peterca M., Wilson D.A., Graf R., Spiess H.W., Balagurusamy V.S.K., Heiney P.A.: J. Am. Chem. Soc. 131, 7662–7677 (2009)
Percec V., Imam M.R., Peterca M., Wilson D.A., Heiney P.A.: J. Am. Chem. Soc. 131, 1294–1304 (2009)
Naidoo K.J., Hughes S.J., Moss J.R.: Macromolecules 32, 331–341 (1999)
Lukin O., Schubert D., Müller C.M., Schweizer W.B., Gramlich V., Schneider J., Dolgonos G., Shivanyuk A.: Proc. Nat. Acad. Sci. 106, 10922–10927 (2009)
E.C. Constable, B.A. Hermann, C.E. Housecroft, L. Merz, L.J. Scherer, Chem. Commun. 928–929 (2004)
Scherer L.J., Merz L., Constable E.C., Housecroft C.E., Neuburger M., Hermann B.A.: J. Am. Chem. Soc. 127, 4033–4041 (2005)
Merz L., Güntherodt H.-J., Scherer L.J., Constable E.C., Housecroft C.E., Hermann B.A.: Chem.–Eur. J. 11, 2307–2318 (2005)
Pan Z., Cheung E.Y., Harris K.D.M., Constable E.C., Housecroft C.E.: Cryst. Grow. Des. 4, 451–455 (2004)
Pan Z., Cheung E.Y., Harris K.D.M., Constable E.C., Housecroft C.E.: Cryst. Grow. Des. 5, 2084–2090 (2005)
Crippen G.M.: J. Math. Chem. 6, 307–324 (1991)
Del Río Echenique G., Rodríguez Schmidt R., Freire J.J., Hernández Cifre J.G., García de la Torre J.: J. Am. Chem. Soc. 131, 8548–8556 (2009)
Speight J.G.: Perry’s Standard Tables and Formulas for Chemical Engineers, pp. 32. McGraw-Hill, New York (2003)
Saalfrank R.W., Deutscher C., Maid H., Ako A.M., Sperner S., Nakajima T., Bauer W., Hampel F., Heß B.A., van Eikema Hommes N.J.R., Puchta R., Heinemann F.W.: Chem. Eur. J. 10, 1899–1905 (2004)
Constable E.C., Hermann B.A., Housecroft C.E., Neuburger M., Schaffner S., Scherer L.J.: New J. Chem. 29, 1475–1481 (2005)
Chen W.-Z., Fanwick P.E., Ren T.: Inorg. Chem. 46, 3429–3431 (2007)
Pawlica D., Marszałek M., Mynarczuk G., Sieroń L., Eilmes J.: New J. Chem. 28, 1615–1621 (2004)
Gibtner T., Hampel F., Gisselbrecht J.-P., Hirsch A.: Chem. Eur. J. 8, 408–432 (2002)
Sánchez-Méndez A., de Jesús E., Flores J.C., Gómez-Sal P.: Inorg. Chem. 46, 4793–4795 (2007)
Yang H.-B., Hawkridge A.M., Huang S.D., Das N., Bunge S.D., Muddiman D.C., Stang P.J.: J. Am. Chem. Soc. 129, 2120–2129 (2007)
Karakaya B., Claussen W., Gessler K., Saenger W., Schlüter A.-D.: J. Am. Chem. Soc. 119, 3296–3301 (1997)
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Eng. Aurelian Niculescu on the occasion of his birthday.
Rights and permissions
About this article
Cite this article
Stadler, AM. Molecular geometry and conformation: mathematical relations between intra-molecular distances and conformation in Fréchet-type dendrimers and dendrons. J Math Chem 48, 566–582 (2010). https://doi.org/10.1007/s10910-010-9692-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-010-9692-4