Abstract
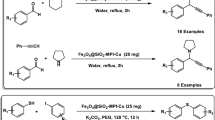
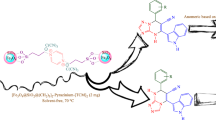
In this research project, we first synthesized the magnetic manganese nanocatalyst by immobilizing the MnCl2 on the surface of magnetic Fe3O4 nanoparticles modified with catechol and piperazine/phenanthroline ligand [Fe3O4@Catechol/PZ-Phen-MnCl2], then we evaluated its catalytic performance in the synthesis of propargyl amines through the A3 and KA2-coupling three-component reactions aromatic aldehydes or ketones, with alkynes, and amines. FT-IR, SEM, TEM, EDX, XRD, TGA, VSM, ICP-OES and elemental mapping analyzes confirmed the successful synthesis of Fe3O4@Catechol/PZ-Phen-MnCl2 nanocatalyst. The results of the coupling reactions confirmed that the Fe3O4@Catechol/PZ-Phen-MnCl2 nanocomposite is a very efficient catalyst for the preparation of various derivatives of propargyl amines. This method has significant features compared to the previous methods, which can be mentioned as follows: synthesis of products with very high efficiency, carrying out the reaction under mild conditions and compatible with the environment, using green and recyclable catalyst, can being applicable to a wide range of substrates and providing NMR analysis for all propargyl amine products.
Graphical Abstract
















Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
R. Upadhyay, R. Rana, S.K. Maurya, ChemCatChem 13, 1867 (2021)
M. Ghobadi, P. PourmoghaddamQhazvini, M. Kazemi, Synth. Commun. 50, 3717 (2020)
K. Banihashemi, M. Sadat, Biol. Mol. Chem. 1, 45 (2023)
M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami, A. Ghorbani-Choghamarani, J. Ind. Eng. Chem. 97, 1 (2021)
M. Norouzi, N. Noormoradi, M. Mohammadi, Nanomagnetic tetraaza (N4 Donor) macrocyclic schiff base complex of copper(II): Synthesis, characterizations, and its catalytic application in click reaction. Nanoscale Adv. 5, 6594–6605 (2023). https://doi.org/10.1039/D3NA00580A
R. Deilam, F. Moeinpour, F.S. Mohseni-Shahri, Monatshefte Für Chemie - Chem. Mon. 151, 1153 (2020)
M.A. Ashraf, Z. Liu, W.-X. Peng, L. Zhou, Catal. Letters 150, 1128 (2020)
D.H. Kim, J. Lee, A. Lee, Org. Lett. 20, 764 (2018)
F. Ghobakhloo, M. Mohammadi, M. Ghaemi, D. Azarifar, Post-synthetic generation of amino-acid-functionalized UiO-66-NH 2 metal–organic framework nanostructures as an amphoteric catalyst for organic reactions, ACS Appl. Nano Mater.7(1), 1265–1277 (2024). https://doi.org/10.1021/acsanm.3c05230
H. Narimani, J. Synth. Chem. 1, 62 (2022)
Z. Moghadasi, A. NooryFajer, A.M.H. Abudken, H. Ali Al-Bahrani, Nanomater. Chem 1, 24 (2023)
L. Chen, A. NooryFajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
S.W.M. Crossley, C. Obradors, R.M. Martinez, R.A. Shenvi, Chem. Rev. 116, 8912 (2016)
Z. Huang, X. Jiang, S. Zhou, P. Yang, C. Du, Y. Li, Chemsuschem 12, 3054 (2019)
B. Zhou, H. Chen, C. Wang, J. Am. Chem. Soc. 135, 1264 (2013)
X. Sun, X. Li, S. Song, Y. Zhu, Y.-F. Liang, N. Jiao, J. Am. Chem. Soc. 137, 6059 (2015)
Y. Yuan, Y. Zheng, B. Xu, J. Liao, F. Bu, S. Wang, J.-G. Hu, A. Lei, ACS Catal. 10, 6676 (2020)
W. Liu, J.T. Groves, Acc. Chem. Res. 48, 1727 (2015)
M. Kazemi, M. Ghobadi, Nanotechnol. Rev. 6, 549 (2017)
M.M. Ba-Abbad, A. Benamour, D. Ewis, A.W. Mohammad, E. Mahmoudi, JOM 74, 3531 (2022)
J. Dadashi, M. Khaleghian, B. Mirtamizdoust, Y. Hanifehpour, S.W. Joo, Crystals 12, 862 (2022)
Y. Riadi, M.M. Kadhim, S. Jawad Shoja, M. Hussein Ali, Y. Fakri Mustafa, A. Sajjadi, Synth. Commun. 52, 875 (2022)
M. Ghazvini, F. Sheikholeslami-Farahani, N.F. Hamedani, A.S. Shahvelayati, Z. Rostami, Comb. Chem. High Throughput Screen. 24, 1261 (2021)
R. Eisavi, F. Ahmadi, Sci. Rep. 12, 11939 (2022)
M. Ma, P. Hou, P. Zhang, J. Cao, H. Liu, H. Yue, G. Tian, S. Feng, Appl. Catal. A Gen. 602, 117709 (2020)
A.N. Fajer, H.A. Al-Bahrani, A.A.H. Kadhum, M. Kazemi, J. Mol. Struct. 1296, 136800 (2024)
S. Jdanova, M.S. Taylor, J. Org. Chem. 88, 3487 (2023)
A.Y. El-Khateeb, S.E. Hamed, K.M. Elattar, RSC Adv. 12, 11808 (2022)
H. Amini, S. Neamani, L. Moradi, ChemistrySelect 6, 9608 (2021)
K. Lauder, A. Toscani, N. Scalacci, D. Castagnolo, Chem. Rev. 117, 14091 (2017)
T.P. Lebold, A.B. Leduc, M.A. Kerr, Org. Lett. 11, 3770 (2009)
Z. He, A.K. Yudin, Angew. Chemie 122, 1651 (2010)
G. Magueur, B. Crousse, D. Bonnet-Delpon, Tetrahedron Lett. 46, 2219 (2005)
X. Sheng, K. Chen, C. Shi, D. Huang, Synthesis (Stuttg). 52, 1 (2020)
Y. Pan, D. Wang, Y. Chen, D. Zhang, W. Liu, X. Yang, ACS Catal. 11, 8443 (2021)
H. Setia Budi, Y. Fakri Mustafa, M.M. Al-Hamdani, A. Surendar, M. Ramezani, Synth. Commun. 51, 3694 (2021)
G. Huang, Z. Yin, X. Zhang, Chem. - A Eur. J. 19, 11992 (2013)
S. Ghosh, K. Biswas, RSC Adv. 11, 2047 (2021)
M. Abedi, M. Hosseini, A. Arabmarkadeh, M. Kazemi, Magnetic nanocatalysts in A3 coupling reactions. Synth. Commun. 51, 835–855 (2021). https://doi.org/10.1080/00397911.2020.1858320
M. Martinez-Amezaga, R.A. Giordano, D.N. Prada Gori, C. PermingeatSquizatto, M.V. Giolito, O.G. Scharovsky, V.R. Rozados, M.J. Rico, E.G. Mata, C.M.L. Delpiccolo, Org. Biomol. Chem. 18, 2475 (2020)
Z. Esam, M. Akhavan, A. Bekhradnia, M. Mohammadi, S. Tourani, Catal. Letters 150, 3112 (2020)
F. Taghavi, M. Gholizadeh, A.S. Saljooghi, M. Ramezani, Medchemcomm 8, 1953 (2017)
N. Bagherzadeh, A.R. Sardarian, H. Eslahi, Sustainable and recyclable magnetic nanocatalyst of 1,10-phenanthroline Pd(0) complex in green synthesis of biaryls and tetrazoles using arylboronic acids as versatile substrates. Mol. Catal. 504, 111489 (2021). https://doi.org/10.1016/j.mcat.2021.111489
E. Yıldırım, B. Arıkan, O. Yücel, O. Çakır, N.T. Kara, T.B. İyim, G. Gürdağ, S. Emik, Chem. Pap. 76, 5747 (2022)
A. Szymczyk, M. Drozd, A. Kamińska, M. Matczuk, M. Trzaskowski, M. Mazurkiewicz-Pawlicka, R. Ziółkowski, E. Malinowska, Int. J. Mol. Sci. 23, 8860 (2022)
B.V. BhaskaraRao, D.P. Pabba, R. Aepuru, A. Akbari-Fakhrabadi, P. Lokhande, R. Udayabhaskar, M. Rosales-Vera, R. Espinoza-González, J. Mater. Sci. Mater. Electron. 34, 1910 (2023)
N. Motahharifar, M. Nasrollahzadeh, A. Taheri-Kafrani, R.S. Varma, M. Shokouhimehr, Magnetic chitosan-copper nanocomposite: A plant assembled catalyst for the synthesis of amino- and N-sulfonyl tetrazoles in eco-friendly media, Carbohydr. Polym. 232, 115819 (2020). https://doi.org/10.1016/j.carbpol.2019.115819
S. Esmaili, A.R. Moosavi-Zare, A. Khazaei, RSC Adv. 12, 5386 (2022)
C. Mukhopadhyay, S. Rana, Catal. Commun. 11, 285 (2009)
K. Jayaramulu, K.K.R. Datta, M.V. Suresh, G. Kumari, R. Datta, C. Narayana, M. Eswaramoorthy, T.K. Maji, ChemPlusChem 77, 743 (2012)
F. Saadati, M. Gholinejad, H. Janmohammadi, S. Shaybanizadeh, Lett. Org. Chem. 15, 1 (2017)
F.M. Moghaddam, S.E. Ayati, S.H. Hosseini, A. Pourjavadi, RSC Adv. 5, 34502 (2015)
M. Kantam, J. Yadav, S. Laha, S. Jha, Synlett 2009, 1791 (2009)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Ying Liu: Performing Experimental works and taking analyzes. Huili Zhang: Performing Experimental works and Manager Project.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Zhang, H. An Attractive and Efficient Method for Preparation of Propargyl Amines: Presentation of a Novel Magnetically Reusable Manganese Nanocatalyst for C-N Bond Formation. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03086-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03086-4