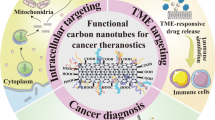
Abstract
The utilization of carbon nanotubes (CNTs) in the field of cancer therapeutics has emerged as a promising avenue of research in recent years. This inquiry delves into the multifaceted potential of bio-functionalized carbon nanotubes for diverse anti-cancer activities. Carbon nanotubes, with their distinct structural and physicochemical properties, offer a versatile platform for innovative approaches in cancer diagnosis, treatment, and comprehension of the underlying molecular mechanisms. The process of bio-functionalizing CNTs plays a pivotal role in enhancing their compatibility with living organisms and specificity, thereby facilitating precise targeting of cancer cells. This scholarly article elucidates the strategies employed for modifying the surface of CNTs and characterizes the bio-functionalized CNTs, setting the stage for their application in anti-cancer therapies. Through a thorough examination of the anti-cancer activities, this research investigates the potential of CNTs to serve as carriers for drug delivery, exemplifying their ability to enhance the availability of drugs and mitigate side effects. Moreover, this study elucidates the diagnostic potential of bio-functionalized CNTs, explicating their applications in cancer imaging, detection, and identification of biomarkers. Mechanistically, the article unveils the pathways through which these nanotubes exert their anti-cancer effects, including the induction of apoptosis and modulation of autophagy. While acknowledging the immense promise of bio-functionalized CNTs, this research also highlights the existing challenges and limitations. Looking toward the future, this article discusses emerging trends in CNT-based cancer therapies, envisioning a future where personalized medicine and nanotechnology converge to revolutionize cancer treatment. Ethical and regulatory considerations are also deliberated upon, emphasizing the need for a responsible and safe transition of these technologies from laboratory experiments to clinical applications. In summary, this study unravels the burgeoning potential of bio-functionalized carbon nanotubes in various anti-cancer activities, underscoring their transformative impact on cancer research and therapy. As the field of nanomedicine continues to expand, these findings contribute to a deeper understanding of the possibilities that lie ahead in the battle against cancer.
Graphical Abstract




Similar content being viewed by others
References
R. Feynman, There’s plenty of room at the bottom Richard Feynman: 1960 [1959], in Nanotechnologie als Kollektivsymbol. ed. by N. Frauke (Transcript Verlag, Bielefeld, 2017), pp.459–470. https://doi.org/10.1515/9783839438039-022
S. Iijima, Helical microtubules of graphitic carbon. Nature 354(6348), 56–58 (1991). https://doi.org/10.1038/354056a0
S. Iijima, T. Ichihashi, Single-shell carbon nanotubes of 1-nm diameter. Nature 363(6430), 603–605 (1993). https://doi.org/10.1038/363603a0
M. Terrones, Science and technology of the twenty-first century: synthesis, properties, and applications of carbon nanotubes. Annu. Rev. Mater. Res. 33(1), 419–501 (2003). https://doi.org/10.1146/annurev.matsci.33.012802.100255
M.S. Dresselhaus, G. Dresselhaus, R. Saito, A. Jorio, Chapter 4 Raman spectroscopy of carbon nanotubes, in Carbon Nanotubes: Quantum Cylinders Of Graphene. ed. by S. Saito, A. Zettl (Elsevier, Amsterdam, 2008), pp.83–108. https://doi.org/10.1016/s1572-0934(08)00004-8
T. Maruyama, Carbon nanotubes, in Handbook of Carbon-Based Nanomaterials. ed. by S. Thomas et al. (Elsevier, Amsterdam, 2021), pp.299–319. https://doi.org/10.1016/b978-0-12-821996-6.00009-9
R.H. Baughman, A.A. Zakhidov, W.A. de Heer, Carbon nanotubes–the route toward applications. Science 297(5582), 787–792 (2002). https://doi.org/10.1126/science.1060928
A. Bianco, K. Kostarelos, C.D. Partidos, M. Prato, Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 5, 571 (2005). https://doi.org/10.1039/b410943k
M.S. Dresselhaus, G. Dresselhaus, A. Jorio, Unusual properties and structure of carbon nanotubes. Annu. Rev. Mater. Res. 34(1), 247–278 (2004). https://doi.org/10.1146/annurev.matsci.34.040203.114607
M.-F. Yu, B.S. Files, S. Arepalli, R.S. Ruoff, Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys. Rev. Lett. 84(24), 5552–5555 (2000). https://doi.org/10.1103/physrevlett.84.5552
M. Meyyappan, L. Delzeit, A. Cassell, D. Hash, Carbon nanotube growth by PECVD: a review. Plasma Sources Sci. Technol. 12(2), 205–216 (2003). https://doi.org/10.1088/0963-0252/12/2/312
T. Lin, V. Bajpai, T. Ji, L. Dai, Chemistry of carbon nanotubes. Aust. J. Chem. 56(7), 635 (2003). https://doi.org/10.1071/ch02254
M. Zhang, J. Li, Carbon nanotube in different shapes. Mater. Today 12(6), 12–18 (2009). https://doi.org/10.1016/s1369-7021(09)70176-2
Z. Liu, S. Tabakman, K. Welsher, H. Dai, Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2(2), 85–120 (2009). https://doi.org/10.1007/s12274-009-9009-8
M. Hiramatsu, M. Hori, Aligned growth of single-walled and double-walled carbon nanotube films by control of catalyst preparation. Carbon Nanotubes – Synth. Charact. Appl. (2011). https://doi.org/10.5772/17657
K. Welsher, Z. Liu, S.P. Sherlock, J.T. Robinson, Z. Chen, D. Daranciang, H. Dai, A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 4(11), 773–780 (2009). https://doi.org/10.1038/nnano.2009.294
X. Zhang, L. Meng, Q. Lu, Z. Fei, P.J. Dyson, Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 30(30), 6041–6047 (2009). https://doi.org/10.1016/j.biomaterials.2009.07.025
R. Singh, S.V. Torti, Carbon nanotubes in hyperthermia therapy. Adv. Drug Deliv. Rev. 65(15), 2045–2060 (2013). https://doi.org/10.1016/j.addr.2013.08.001
F. Zhou, D. Xing, Z. Ou, B. Wu, D.E. Resasco, W.R. Chen, Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J. Biomed. Opt. 14(2), 021009 (2009). https://doi.org/10.1117/1.3078803
S. Xie, W. Li, Z. Pan, B. Chang, L. Sun, Mechanical and physical properties on carbon nanotube. J. Phys. Chem. Solids 61(7), 1153–1158 (2000). https://doi.org/10.1016/s0022-3697(99)00376-5
J.A. Elliott, J.K.W. Sandler, A.H. Windle, R.J. Young, M.S.P. Shaffer, Collapse of single-wall carbon nanotubes is diameter dependent. Phys. Rev. Lett. (2004). https://doi.org/10.1103/physrevlett.92.095501
N. Hamada, S. Sawada, A. Oshiyama, New one-dimensional conductors: graphitic microtubules. Phys. Rev. Lett. 68(10), 1579–1581 (1992). https://doi.org/10.1103/physrevlett.68.1579
R. Saito, M. Fujita, G. Dresselhaus, M.S. Dresselhaus, Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 60(18), 2204–2206 (1992). https://doi.org/10.1063/1.107080
J.W.G. Wilder, L.C. Venema, A.G. Rinzler, R.E. Smalley, C. Dekker, Electronic structure of atomically resolved carbon nanotubes. Nature 391(6662), 59–62 (1998). https://doi.org/10.1038/34139
T.W. Odom, J.-L. Huang, P. Kim, C.M. Lieber, Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 391(6662), 62–64 (1998). https://doi.org/10.1038/34145
C.M. Lieber, One-dimensional nanostructures: chemistry, physics & applications. Solid State Commun. 107(11), 607–616 (1998). https://doi.org/10.1016/s0038-1098(98)00209-9
M.S. Dresselhaus, G. Dresselhaus, R. Saito, A. Jorio, Raman spectroscopy of carbon nanotubes. Phys. Rep. 409(2), 47–99 (2005). https://doi.org/10.1016/j.physrep.2004.10.006
V. Neves, E. Heister, S. Costa, C. Tîlmaciu, E. Flahaut, B. Soula, H.M. Coley, J. McFadden, S.R.P. Silva, Design of double-walled carbon nanotubes for biomedical applications. Nanotechnology 23(36), 365102 (2012). https://doi.org/10.1088/0957-4484/23/36/365102
M. Monthioux, P. Serp, B. Caussat, E. Flahaut, M. Razafinimanana, F. Valensi, C. Laurent, A. Peigney, D. Mesguich, A. Weibel, W. Bacsa, J.M. Broto, Carbon nanotubes, in Springer Handbook of Nanotechnology. ed. by B. Bhushan (Springer, Berlin, 2017), pp.193–247. https://doi.org/10.1007/978-3-662-54357-3_8
B.P. Grady, The use of solution viscosity to characterize single-walled carbon nanotube dispersions. Macromol. Chem. Phys. 207(23), 2167–2169 (2006). https://doi.org/10.1002/macp.200600473
H. Gong, R. Peng, Z. Liu, Carbon nanotubes for biomedical imaging: the recent advances. Adv. Drug Deliv. Rev. 65(15), 1951–1963 (2013). https://doi.org/10.1016/j.addr.2013.10.002
N. Punbusayakul, S. Talapatra, P.M. Ajayan, W. Surareungchai, Label-free as-grown double wall carbon nanotubes bundles for Salmonella typhimuriumimmunoassay. Chem. Cent. J. (2013). https://doi.org/10.1186/1752-153x-7-102
I. Ojeda, M. Barrejón, L.M. Arellano, A. González-Cortés, P. Yáñez-Sedeño, F. Langa, J.M. Pingarrón, Grafted-double walled carbon nanotubes as electrochemical platforms for immobilization of antibodies using a metallic-complex chelating polymer: application to the determination of adiponectin cytokine in serum. Biosens. Bioelectron. 74, 24–29 (2015). https://doi.org/10.1016/j.bios.2015.06.001
F. Kong, F. Liu, W. Li, X. Guo, Z. Wang, H. Zhang, Q. Li, L. Luo, Y. Du, Y. Jin, J. You, Smart carbon nanotubes with laser-controlled behavior in gene delivery and therapy through a non-digestive trafficking pathway. Small 12(48), 6753–6766 (2016). https://doi.org/10.1002/smll.201601092
A. Burke, X. Ding, R. Singh, R.A. Kraft, N. Levi-Polyachenko, M.N. Rylander, C. Szot, C. Buchanan, J. Whitney, J. Fisher, H.C. Hatcher, R. D’Agostino Jr., N.D. Kock, P.M. Ajayan, D.L. Carroll, S. Akman, F.M. Torti, S.V. Torti, Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc. Natl. Acad. Sci. 106(31), 12897–12902 (2009). https://doi.org/10.1073/pnas.0905195106
C. Wang, L. Xu, C. Liang, J. Xiang, R. Peng, Z. Liu, Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-ctla-4 therapy to inhibit cancer metastasis. Adv. Mater. 26(48), 8154–8162 (2014). https://doi.org/10.1002/adma.201402996
S. Wang, Q. Lin, J. Chen, H. Gao, D. Fu, S. Shen, Biocompatible polydopamine-encapsulated gadolinium-loaded carbon nanotubes for MRI and color mapping guided photothermal dissection of tumor metastasis. Carbon 112, 53–62 (2017). https://doi.org/10.1016/j.carbon.2016.10.096
Z. Liu, A.C. Fan, K. Rakhra, S. Sherlock, A. Goodwin, X. Chen, Q. Yang, D.W. Felsher, H. Dai, Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew. Chem. 121(41), 7804–7808 (2009). https://doi.org/10.1002/ange.200902612
Y. Zhu, Q. Sun, Y. Liu, T. Ma, L. Su, S. Liu, X. Shi, D. Han, F. Liang, Decorating gold nanostars with multiwalled carbon nanotubes for photothermal therapy. R. Soc. Open Sci. 5(8), 180159 (2018). https://doi.org/10.1098/rsos.180159
E. Bekyarova, Y. Ni, E.B. Malarkey, V. Montana, J.L. McWilliams, R.C. Haddon, V. Parpura, Applications of carbon nanotubes in biotechnology and biomedicine. J. Biomed. Nanotechnol. 1(1), 3–17 (2005). https://doi.org/10.1166/jbn.2005.004
C. Journet, P. Bernier, Production of carbon nanotubes. Appl. Phys. A Mater. Sci. Process. 67(1), 1–9 (1998). https://doi.org/10.1007/s003390050731
M.A. Correa-Duarte, N. Wagner, J. Rojas-Chapana, C. Morsczeck, M. Thie, M. Giersig, Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett. 4(11), 2233–2236 (2004). https://doi.org/10.1021/nl048574f
S. Detriche, G. Zorzini, J.-F. Colomer, A. Fonseca, J.B. Nagy, Application of the hansen solubility parameters theory to carbon nanotubes. J. Nanosci. Nanotechnol. 8(11), 6082–6092 (2008). https://doi.org/10.1166/jnn.2008.sw16
P. Wick, P. Manser, L. Limbach, U. Dettlaffweglikowska, F. Krumeich, S. Roth, W. Stark, A. Bruinink, The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 168(2), 121–131 (2007). https://doi.org/10.1016/j.toxlet.2006.08.019
T. Coccini, E. Roda, D.A. Sarigiannis, P. Mustarelli, E. Quartarone, A. Profumo, L. Manzo, Effects of water-soluble functionalized multi-walled carbon nanotubes examined by different cytotoxicity methods in human astrocyte D384 and lung A549 cells. Toxicology 269(1), 41–53 (2010). https://doi.org/10.1016/j.tox.2010.01.005
H. Dai, Carbon nanotubes: synthesis, integration, and properties. Acc. Chem. Res. 35(12), 1035–1044 (2002). https://doi.org/10.1021/ar0101640
U.S. Shin, I.K. Yoon, G.S. Lee, W.C. Jang, J.C. Knowles, H.W. Kim, Carbon nanotubes in nanocomposites and hybrids with hydroxyapatite for bone replacements. J. Tissue Eng. (2011). https://doi.org/10.4061/2011/674287
H. Hu, Y. Ni, V. Montana, R.C. Haddon, V. Parpura, Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 4(3), 507–511 (2004). https://doi.org/10.1021/nl035193d
A. Sucapane, G. Cellot, M. Prato, M. Giugliano, V. Parpura, L. Ballerini, Interactions between cultured neurons and carbon nanotubes: a nanoneuroscience vignette. J. Nanoneurosci. 1(1), 10–16 (2009). https://doi.org/10.1166/jns.2009.002
J.L. Gilmore, X. Yi, L. Quan, A.V. Kabanov, Novel nanomaterials for clinical neuroscience. J. Neuroimmune Pharmacol. 3(2), 83–94 (2008). https://doi.org/10.1007/s11481-007-9099-6
A. Nunes, N. Amsharov, C. Guo, J. Van den Bossche, P. Santhosh, T.K. Karachalios, S.F. Nitodas, M. Burghard, K. Kostarelos, K.T. Al-Jamal, Hybrid polymer-grafted multiwalled carbon nanotubes for in vitro gene delivery. Small 6(20), 2281–2291 (2010). https://doi.org/10.1002/smll.201000864
E. Jan, N.A. Kotov, Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 7(5), 1123–1128 (2007). https://doi.org/10.1021/nl0620132
T.I. Chao, S. Xiang, C.S. Chen, W.C. Chin, A.J. Nelson, C. Wang, J. Lu, Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem. Biophys. Res. Commun. 384(4), 426–430 (2009). https://doi.org/10.1016/j.bbrc.2009.04.157
Y. Ni, H. Hu, E.B. Malarkey, B. Zhao, V. Montana, R.C. Haddon, V. Parpura, Chemically functionalized water soluble single-walled carbon nanotubes modulate neurite outgrowth. J. Nanosci. Nanotechnol. 5(10), 1707–1712 (2005). https://doi.org/10.1166/jnn.2005.189
F.M. Xu, J.P. Xu, J. Ji, J.C. Shen, A novel biomimetic polymer as amphiphilic surfactant for soluble and biocompatible carbon nanotubes (CNTs). Colloids Surf. B 67(1), 67–72 (2008). https://doi.org/10.1016/j.colsurfb.2008.07.016
E.B. Malarkey, K.A. Fisher, E. Bekyarova, W. Liu, R.C. Haddon, V. Parpura, Conductive single-walled carbon nanotube substrates modulate neuronal growth. Nano Lett. 9(1), 264–268 (2009). https://doi.org/10.1021/nl802855c
Z. Liu, S.M. Tabakman, Z. Chen, H. Dai, Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 4(9), 1372–1381 (2009). https://doi.org/10.1038/nprot.2009.146
Y. Zhang, Y. Xu, Z. Li, T. Chen, S.M. Lantz, P.C. Howard, M.G. Paule, W. Slikker Jr., F. Watanabe, T. Mustafa, A.S. Biris, S.F. Ali, Mechanistic toxicity evaluation of uncoated and pegylated single-walled carbon nanotubes in neuronal PC12 cells. ACS Nano 5(9), 7020–7033 (2011). https://doi.org/10.1021/nn2016259
J.A. Roman, T.L. Niedzielko, R.C. Haddon, V. Parpura, C.L. Floyd, Single-Walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J. Neurotrauma 28(11), 2349–2362 (2011). https://doi.org/10.1089/neu.2010.1409
A.T. Woolley, Methods Mol. Biol. 283, 305–319 (2004)
M. Foldvari, M. Bagonluri, Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomed. Nanotechnol. Biol. Med. 4(3), 183–200 (2008). https://doi.org/10.1016/j.nano.2008.04.003
A.M. Smith, S. Nie, Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc. Chem. Res. 43(2), 190–200 (2009). https://doi.org/10.1021/ar9001069
V. Leiro, P. Parreira, S.C. Freitas, M.C.L. Martins, A.P. Pêgo, Conjugation chemistry principles and surface functionalization of nanomaterials, in Biomedical Applications of Functionalized Nanomaterials. ed. by B. Sarmento, J. das Neves (Elsevier, Amsterdam, 2018), pp.35–66. https://doi.org/10.1016/b978-0-323-50878-0.00002-1
Q. Zhao, Y. Gao, Surface modification and functionalization of nanomaterials for biomedical applications, in Functionalized Nanomaterials for the Management of Microbial Infection. ed. by R. Boukherroub, S. Szunerits, D. Drider (Elsevier, Amsterdam, 2016), pp.117–137
B. Sitharaman, Nanotechnology in cancer therapy: a biocompatible approach. J. Bionanosci. 5(4), 294–312 (2011)
L. Li, W. Jiang, K. Luo, H. Song, F. Lan, Y. Wu, Z. Gu, Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 3(8), 595–615 (2013). https://doi.org/10.7150/thno.5366
K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato, A. Bianco, Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2(2), 108–113 (2007). https://doi.org/10.1038/nnano.2006.209
J. Wang, Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17(1), 7–14 (2005). https://doi.org/10.1002/elan.200403113
W. Yang, K.R. Ratinac, S.P. Ringer, P. Thordarson, J.J. Gooding, F. Braet, Carbon nanomaterials in biosensors: should you use nanotubes or graphene? Angew. Chem. Int. Ed. 49(12), 2114–2138 (2010). https://doi.org/10.1002/anie.200903463
S.N. Kim, J.F. Rusling, F. Papadimitrakopoulos, Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv. Mater. 19(20), 3214–3228 (2007). https://doi.org/10.1002/adma.200700665
S. Vardharajula, S.Z. Ali, P.M. Tiwari, E. Eroğlu, K. Vig, V.A. Dennis, S.R. Singh, Functionalized carbon nanotubes: biomedical applications. Int. J. Nanomed. (2012). https://doi.org/10.2147/ijn.s35832
K. Kamil Reza, S. Srivastava, S.K. Yadav, A.M. Biradar, Biofunctionalized carbon nanotubes platform for biomedical applications. Mater. Lett. 126, 126–130 (2014). https://doi.org/10.1016/j.matlet.2014.04.017
S. Jain, V.S. Thakare, M. Das, C. Godugu, A.K. Jain, R. Mathur, K. Chuttani, A.K. Mishra, Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem. Res. Toxicol. 24(11), 2028–2039 (2011). https://doi.org/10.1021/tx2003728
K. Balasubramanian, M. Burghard, Chemically functionalized carbon nanotubes. Small 1(2), 180–192 (2005). https://doi.org/10.1002/smll.200400118
Z. Liu, X. Sun, N. Nakayama-Ratchford, H. Dai, Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 1(1), 50–56 (2007). https://doi.org/10.1021/nn700040t
I. Mfouo Tynga, H. Abrahamse, Nano-Mediated photodynamic therapy for cancer: enhancement of cancer specificity and therapeutic effects. Nanomaterials 8(11), 923 (2018). https://doi.org/10.3390/nano8110923
M. Prasad, U.P. Lambe, B. Brar, I. Shah, J. Manimegalai, K. Ranjan, R. Rao, S. Kumar, S. Mahant, S.K. Khurana, H. Iqbal, K. Dhama, J. Misri, G. Prasad, Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 97, 1521–1537 (2018). https://doi.org/10.1016/j.biopha.2017.11.026
A. Ediriwickrema, W.M. Saltzman, Nanotherapy for cancer: targeting and multifunctionality in the future of cancer therapies. ACS Biomater. Sci. Eng. 1(2), 64–78 (2015). https://doi.org/10.1021/ab500084g
G.S.R. Raju, B. Dariya, S.K. Mungamuri, G. Chalikonda, S.-M. Kang, I.N. Khan, P.S. Sushma, G.P. Nagaraju, E. Pavitra, Y.-K. Han, Nanomaterials multifunctional behavior for enlightened cancer therapeutics. Semin. Cancer Biol. 69, 178–189 (2021). https://doi.org/10.1016/j.semcancer.2019.08.013
M.C. Daniel, Drug delivery carriers, in Emerging Applications of Colloidal Noble Metals in Cancer Nanomedicine. ed. by J.R. Lakowicz, J. Zhang (Future Medicine Ltd, London, 2012), pp.54–67. https://doi.org/10.2217/ebo.12.39
K. Maier-Hauff, F. Ulrich, D. Nestler, H. Niehoff, P. Wust, B. Thiesen, H. Orawa, V. Budach, A. Jordan, Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 103(2), 317–324 (2010). https://doi.org/10.1007/s11060-010-0389-0
O.C. Farokhzad, R. Langer, Impact of nanotechnology on drug delivery. ACS Nano 3(1), 16–20 (2009). https://doi.org/10.1021/nn900002m
Z. Liu, C. Davis, W. Cai, L. He, X. Chen, H. Dai, Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. 105(5), 1410–1415 (2008). https://doi.org/10.1073/pnas.0707654105
M.E. Davis, J.E. Zuckerman, C.H.J. Choi, D. Seligson, A. Tolcher, C.A. Alabi, Y. Yen, J.D. Heidel, A. Ribas, Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464(7291), 1067–1070 (2010). https://doi.org/10.1038/nature08956
X. Michalet, F.F. Pinaud, L.A. Bentolila, J.M. Tsay, S. Doose, J.J. Li, G. Sundaresan, A.M. Wu, S.S. Gambhir, S. Weiss, Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307(5709), 538–544 (2005). https://doi.org/10.1126/science.1104274
S. Selvakumar, T. Rajendiran, K. Biswas, Current advances on biomedical applications and toxicity of mwcnts: a review. BioNanoScience 13(2), 860–878 (2023). https://doi.org/10.1007/s12668-023-01110-4
G. Seeta Rama Raju, L. Benton, E. Pavitra, J.S. Yu, Multifunctional nanoparticles: recent progress in cancer therapeutics. Chem. Commun. 51(68), 13248–13259 (2015). https://doi.org/10.1039/c5cc04643b
A. Stojadinovic, I. Avital, G.E. Peoples, S. Steele, Special issue on current challenges and future directions in monitoring recurrence after treatment of primary cancer. J. Cancer 5(4), 260–261 (2014). https://doi.org/10.7150/jca.9070
J. Zugazagoitia, C. Guedes, S. Ponce, I. Ferrer, S. Molina-Pinelo, L. Paz-Ares, Current challenges in cancer treatment. Clin. Ther. 38(7), 1551–1566 (2016). https://doi.org/10.1016/j.clinthera.2016.03.026
J.A. Ludwig, J.N. Weinstein, Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer 5(11), 845–856 (2005). https://doi.org/10.1038/nrc1739
N. Kamaly, Z. Xiao, P.M. Valencia, A.F. Radovic-Moreno, O.C. Farokhzad, Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41(7), 2971 (2012). https://doi.org/10.1039/c2cs15344k
K.K. Jain, Nanotechnology in clinical laboratory diagnostics. Clin. Chim. Acta 358(1–2), 37–54 (2005). https://doi.org/10.1016/j.cccn.2005.03.014
Z. Liu, K. Chen, C. Davis, S. Sherlock, Q. Cao, X. Chen, H. Dai, Drug Delivery with carbon nanotubes for in vivo cancer treatment. Can. Res. 68(16), 6652–6660 (2008). https://doi.org/10.1158/0008-5472.can-08-1468
A.A. Bhirde, V. Patel, J. Gavard, G. Zhang, A.A. Sousa, A. Masedunskas, R.D. Leapman, R. Weigert, J.S. Gutkind, J.F. Rusling, Targeted killing of cancer cells in vivo and in vitro with egf-directed carbon nanotube-based drug delivery. ACS Nano 3(2), 307–316 (2009). https://doi.org/10.1021/nn800551s
S. Prabhu, P. Ananthanarayanan, S.K. Aziz, S. Rai, S. Mutalik, S.R.B. Sadashiva, Enhanced effect of geldanamycin nanocomposite against breast cancer cells growing in vitro and as xenograft with vanquished normal cell toxicity. Toxicol. Appl. Pharmacol. 320, 6072 (2017)
M. Rasoulzadeh, H. Namazi, Carboxymethyl cellulose/graphene oxide bionanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 168, 320326 (2017)
R. Dhivya, J. Ranjani, P.K. Bowen, J. Rajendhran, J. Mayandi, J. Annaraj, Biocompatible curcumin loaded PMMA-PEG/ZnO nanocomposite induce apoptosis and cytotoxicity in human gastric cancer cells. Mater. Sci. Eng. C 80, 59–68 (2017). https://doi.org/10.1016/j.msec.2017.05.128
E.A. Nivethaa, S. Dhanavel, A. Rebekah, V. Narayanan, A. Stephen, A comparative study of 5-Fluorouracil release from chitosan/silver and chitosan/silver/MWCNT nanocomposites and their cytotoxicity towards MCF-7. Mater. Sci. Eng. C 66, 244–250 (2016). https://doi.org/10.1016/j.msec.2016.04.080
L. Zhang, K. Xia, Z. Lu, X. Shuai, Targeted therapy for cancer with prostate-specific membrane antigen (PSMA) and survivin dual-targeted gold nanoparticles. Eur. J. Pharm. Biopharm. 107, 174–181 (2016)
D. Peer, J.M. Karp, S. Hong, O.C. Farokhzad, R. Margalit, R. Langer, Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2(12), 751–760 (2007). https://doi.org/10.1038/nnano.2007.387
J. Shi, P.W. Kantoff, R. Wooster, O.C. Farokhzad, Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17(1), 20–37 (2016). https://doi.org/10.1038/nrc.2016.108
A. Jasim, S. Abdelghany, K. Greish, Current update on the role of enhanced permeability and retention effect in cancer nanomedicine, in Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. ed. by V. Mishra et al. (Elsevier, 2017), pp.62–109. https://doi.org/10.1016/b978-0-12-809717-5.00002-6
Q. Zhang, Y. Zhang, Y. Ji, Leveraging the enhanced permeability and retention effect for tumor targeting nanomedicine. J. Nanomater. 2017, 1–12 (2017)
M. Ferrari, Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5(3), 161–171 (2005). https://doi.org/10.1038/nrc1566
O. Tacar, P. Sriamornsak, C.R. Dass, Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 65(2), 157–170 (2012). https://doi.org/10.1111/j.2042-7158.2012.01567.x
A. Wicki, D. Witzigmann, V. Balasubramanian, J. Huwyler, Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J. Control. Release 200, 138–157 (2015). https://doi.org/10.1016/j.jconrel.2014.12.030
H. Maeda, Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 91, 3–6 (2015). https://doi.org/10.1016/j.addr.2015.01.002
J. Hrkach, D. Von Hoff, M.M. Ali, E. Andrianova, J. Auer, T. Campbell, D. De Witt, M. Figa, M. Figueiredo, A. Horhota, S. Low, K. McDonnell, E. Peeke, B. Retnarajan, A. Sabnis, E. Schnipper, J.J. Song, Y.H. Song, J. Summa et al., Preclinical development and clinical translation of a psma-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. (2012). https://doi.org/10.1126/scitranslmed.3003651
K.C. Anderson, Progress and paradigms in multiple myeloma. Clin. Cancer Res. 22(22), 5419–5427 (2016). https://doi.org/10.1158/1078-0432.ccr-16-0625
S.V. Rajkumar, Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 91(7), 719–734 (2016). https://doi.org/10.1002/ajh.24402
N. Raje, J. Berdeja, Y. Lin, D. Siegel, S. Jagannath, D. Madduri, M. Liedtke, J. Rosenblatt, M.V. Maus, A. Turka, L.-P. Lam, R.A. Morgan, K. Friedman, M. Massaro, J. Wang, G. Russotti, Z. Yang, T. Campbell, K. Hege et al., Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380(18), 1726–1737 (2019). https://doi.org/10.1056/nejmoa1817226
M. Mohammadi, M. Salavati-Niasari, A review on the recent progress, challenges and perspectives of self-healing nanocomposite hydrogels. Polymer 211, 123098 (2020)
F. Cheng, H. Chen, H. Li, Recent advances in tough and self-healing nanocomposite hydrogels for shape morphing and soft actuators. Eur. Polymer J. 124, 109448 (2020). https://doi.org/10.1016/j.eurpolymj.2019.109448
B.W. Smith, M. Monthioux, D.E. Luzzi, Encapsulated C60 in carbon nanotubes. Nature 396(6709), 323–324 (1998). https://doi.org/10.1038/24521
R.J. Chen, Y. Zhang, D. Wang, H. Dai, Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 123(16), 3838–3839 (2001). https://doi.org/10.1021/ja010172b
J. Li, Y. Lu, E.S. Yeung, Quantitative analysis of single-molecule DNA bending. Analyst 127(7), 908–912 (2002)
Z. Wang, M. Gerstein, M. Snyder, RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10(1), 57–63 (2009). https://doi.org/10.1038/nrg2484
R. Aebersold, M. Mann, Mass spectrometry-based proteomics. Nature 422(6928), 198–207 (2003). https://doi.org/10.1038/nature01511
E.F. Petricoin III., A.M. Ardekani, B.A. Hitt, P.J. Levine, V.A. Fusaro, S.M. Steinberg, G.B. Mills, C. Simone, D.A. Fishman, E.C. Kohn, L.A. Liotta, Use of proteomic patterns in serum to identify ovarian cancer. Lancet 359(9306), 572–577 (2002). https://doi.org/10.1016/s0140-6736(02)07746-2
J. Shendure, G.J. Porreca, N.B. Reppas, X. Lin, J.P. McCutcheon, A.M. Rosenbaum, M.D. Wang, K. Zhang, R.D. Mitra, G.M. Church, Accurate multiplex polony sequencing of an evolved bacterial genome. Science 309(5741), 1728–1732 (2005). https://doi.org/10.1126/science.1117389
J.L. Spratlin, N.J. Serkova, S.G. Eckhardt, Clinical applications of metabolomics in oncology: a review. Clin. Cancer Res. 15(2), 431–440 (2009). https://doi.org/10.1158/1078-0432.ccr-08-1059
M.V. Berridge, P.M. Herst, A.S. Tan, Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction, in Biotechnology Annual Review. ed. by M.R. El-Gewely (Elsevier, Amsterdam, 2005), pp.127–152. https://doi.org/10.1016/s1387-2656(05)11004-7
J. Lu, G. Getz, E.A. Miska, E. Alvarez-Saavedra, J. Lamb, D. Peck, A. Sweet-Cordero, B.L. Ebert, R.H. Mak, A.A. Ferrando, J.R. Downing, T. Jacks, H.R. Horvitz, T.R. Golub, MicroRNA expression profiles classify human cancers. Nature 435(7043), 834–838 (2005). https://doi.org/10.1038/nature03702
R.L. Wahl, H. Jacene, Y. Kasamon, M.A. Lodge, From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50(Suppl 1), 122S-150S (2009). https://doi.org/10.2967/jnumed.108.057307
J.C.M. Wan, C. Massie, J. Garcia-Corbacho, F. Mouliere, J.D. Brenton, C. Caldas, S. Pacey, R. Baird, N. Rosenfeld, Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17(4), 223–238 (2017). https://doi.org/10.1038/nrc.2017.7
E. Wang, J. Zou, N. Zaman, L.K. Beitel, M. Trifiro, M. Paliouras, Cancer systems biology in the genome sequencing era: Part 1, dissecting and modeling of tumor clones and their networks. Semin. Cancer Biol. 23(4), 279–285 (2013). https://doi.org/10.1016/j.semcancer.2013.06.002
C.H. Xia, W.X. Yu, B.Q. Wang, Y. Wang, & L. Wang, The primary mechanism of photoexcited tio2 nanoparticles-induced apoptosis in human hepatoma bel-7402 cells. 2008 International Conference on BioMedical Engineering and Informatics, 2008 https://doi.org/10.1109/bmei.2008.158
X. Wang, Z. Liu, Z. Yu, Research on MWCNTs induced apoptosis of BEL-7402 cells in vitro and the mechanism. Colloids Surf. B 82(1), 41–47 (2011)
B. Levine, G. Kroemer, Autophagy in the pathogenesis of disease. Cell 132(1), 27–42 (2008). https://doi.org/10.1016/j.cell.2007.12.018
R.K. Amaravadi, A.C. Kimmelman, J. Debnath, Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 9(9), 1167–1181 (2019). https://doi.org/10.1158/2159-8290.cd-19-0292
J. Chen, S. Chen, X. Zhao, L.V. Kuznetsova, S.S. Wong, I. Ojima, Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J. Am. Chem. Soc. 130(49), 16778–16785 (2008). https://doi.org/10.1021/ja805570f
J. Meng, M. Yang, F. Jia, Z. Xu, H. Kong, H. Xu, Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology 5(4), 583–591 (2010). https://doi.org/10.3109/17435390.2010.523483
Y. Zhu, B. Zhang, G. Liu, J. He, M. Li, X. Zeng, A PEGylated fluoranthene polymer encapsulated NIR nanosystem with a high capacity for thermal ablation and photothermal therapy of cancer cells in vitro. J. Mater. Chem. 21(32), 12003–12010 (2011)
A.Z. Wang, V. Bagalkot, C.C. Vasilliou, F. Gu, F. Alexis, L. Zhang, M. Shaikh, K. Yuet, M.J. Cima, R. Langer, P.W. Kantoff, N.H. Bander, S. Jon, O.C. Farokhzad, Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. ChemMedChem 3(9), 1311–1315 (2008). https://doi.org/10.1002/cmdc.200800091
X. Wang, T. Xia, S. Addo Ntim, Z. Ji, S. Lin, H. Meng, C.H. Chung, S. George, H. Zhang, M. Wang, N. Li, Y. Yang, V. Castranova, S. Mitra, J.C. Bonner, A.E. Nel, Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano 5(12), 9772–9787 (2011). https://doi.org/10.1021/nn2033055
F. Chen, H. G. Evans, & P.R. Choo, Safety and health considerations for the use of nanomaterials in the construction industry. In Nanotechnology in Construction 3 (Springer, 2013), pp. 27–34
X. Chen, U.C. Tam, J.L. Czlapinski, G.S. Lee, D. Rabuka, A. Zettl, C.R. Bertozzi, Interfacing carbon nanotubes with living cells. J. Am. Chem. Soc. 128(19), 6292–6293 (2006). https://doi.org/10.1021/ja060276s
L. Chen, H. Xie, W. Yu, Functionalization methods of carbon nanotubes and its applications. Carbon Nanotubes Appl. Electron Dev. (2011). https://doi.org/10.5772/18547
J. Chen, S. Chen, X. Zhao, L. Kuznetsova, S.S. Wong, I. Ojima, Functionalization of carbon nanotubes for potential therapeutic applications. Nanoscale Res. Lett. 6(1), 571 (2011)
F. Chen, H.G. Evans, In vitro studies on the biomolecular corona of gold nanoparticles. Toxicol. Lett. 222(3), 289–293 (2013)
Acknowledgements
The authors express their gratitude to the Department of Biotechnology, Sathyabama Institute of Science and Technology, Chennai, as well as the International Research Centre, Centre for Nanoscience and Nanotechnology, SIST, Chennai, for conducting the research.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors made contributions to the conceptualization and design of the study. GBR: were responsible for the preparation of materials, collection and analysis of data, PS: drafted the work and revised it critically for important intellectual content and KB: drafted and approved the version to be published. The final version of the manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no financial or non-financial interests to disclose.
Ethical Approval
This is an observational study therefore no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramesh, G.B., Singh, P. & Biswas, K. Potentialities of Bio-functionalized Carbon Nanotubes for Different Anti-cancerous Activities. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03012-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03012-8