Abstract
This study was designed to improve bioavailability and therapeutic efficacy of Gemcitabine (GEM) with reduced side effects using MOF MIL-100 as cargo. MIL-100 was synthesized, and characterized by microscopic and spectroscopic techniques. Impregnation approach was used for encapsulation of GEM inside the MIL-100 (i.e., MIL100-GEM). In-vitro release studies of MIL100-GEM was carried out in different media (PBS, deionized water and Tris buffer, pH = 7.4, 9.5 mM) to find out the drug release mechanism. Cytotoxicity and apoptosis assays were evaluated using MTT and fluorescence-activated cell sorting (FACS) assay in MiaPaCa-2 pancreatic cancer cell lines. Biocompatibility, pharmacokinetic and biodistribution studies of MIL100-GEM were assessed in Wistar rats. MIL100-GEM exhibited high encapsulation efficiency (78.6 ± 0.5%) and maximum payload (23.6 ± 1%). PXRD confirmed crystallinity of MIL-100, and did not show any effect on its structural integrity after encapsulation of GEM. In-vitro release studies revealed a biphasic release pattern in PBS buffer which followed Higuchi diffusion kinetics. In-vitro cytotoxicity studies showed low IC50 value for MIL100-GEM (3.50 ± 1.33 µg/ml) compared to GEM (6.22 ± 1.55 µg/ml), ensuring adequate cell proliferation after 72 h. Hemolysis study showed that MIL100-GEM (14.54 ± 1.3%) had better biocompatibility than the native GEM (30.52 ± 1.67%). Furthermore, pharmacokinetic and biodistribution studies exhibited ∼17-fold increased bioavailability, ∼20-fold increased distribution half-life and ∼15-folds elimination half-life of GEM with less accumulation of drug in the kidneys. MIL-100 MOF was synthesized and characterized to address the metabolic degradation issue of GEM. Biocompatible, MIL100-GEM demonstrated efficient drug (GEM) loading and enhanced cytotoxic activity in pancreatic cancer cell line with augmented bioavailability, providing MIL-100 a promising drug cargo.
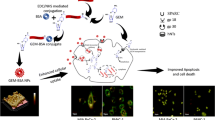
Graphic Abstract
MIL-100 was synthesized using a microwave-assisted method. The anticancer drug Gemcitabine Hydrochloride (GEM) was loaded into the MIL-100 using the impregnation method. Encapsulation protected the drug from its metabolic inactivation to enhance bioavailability in target organs and reduce side effects.










Similar content being viewed by others
References
B. Haley, E. Frenkel, Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 26(1), 57–64 (2008)
K. Tsuchida, Drug delivery systems for cancer treatment, in Encyclopedia of cancer, ed. by M. Schwab (Springer, Berlin, 2011), pp. 1160–1162
C. Bornmann, R. Graeser, N. Esser et al., A new liposomal formulation of Gemcitabine is active in an orthotopic mouse model of pancreatic cancer accessible to bioluminescence imaging. Cancer Chemother. Pharmacol. 61(3), 395–405 (2008)
L.W. Hertel, G.B. Boder, J.S. Kroin et al., Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res. 50(14), 4417–4422 (1990)
W. Plunkett, P. Huang, V. Gandhi, Preclinical characteristics of gemcitabine. Anticancer Drugs 6, 7–13 (1995)
H.A. Burris III, M.J. Moore, J. Andersen et al., Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15(6), 2403–2413 (1997)
J.R. Mackey, R.S. Mani, M. Selner et al., Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 58(19), 4349–4357 (1998)
M. Pastor-Anglada, M. Molina-Arcas, F.J. Casado, B. Bellosillo, D. Colomer, J. Gil, Nucleoside transporters in chronic lymphocytic leukaemia. Leukemia 18(3), 385–393 (2004)
H. Gourdeau, M.L. Clarke, F. Ouellet et al., Mechanisms of uptake and resistance to troxacitabine, a novel deoxycytidine nucleoside analogue, in human leukemic and solid tumor cell lines. Cancer Res. 61(19), 7217–7224 (2001)
J.L. Abbruzzese, R. Grunewald, E.A. Weeks et al., A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J. Clin Oncol. 9(3), 491–498 (1991)
J.M. Reid, W. Qu, S.L. Safgren et al., Phase I trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J. Clin. Oncol. 22(12), 2445–2451 (2004)
R. Moog, A.M. Burger, M. Brandl et al., Change in pharmacokinetic and pharmacodynamic behavior of gemcitabine in human tumor xenografts upon entrapment in vesicular phospholipid gels. Cancer Chemother. Pharmacol. 49(5), 356–366 (2002)
M.L. Immordino, P. Brusa, F. Rocco, S. Arpicco, M. Ceruti, L. Cattel, Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Control. Rel. 100(3), 331–346 (2004)
K. Derakhshandeh, S. Fathi, Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int. J. Pharm. 437(1–2), 172–177 (2012)
J. Yang, H. Lee, W. Hyung, S.B. Park, S. Haam, Magnetic PECA nanoparticles as drug carriers for targeted delivery: synthesis and release characteristics. J. Microencapsul. 23(2), 203–212 (2006)
J. Wang, X. Zhang, Y. Cen, X. Lin, Q. Wu, Antitumor gemcitabine conjugated micelles from amphiphilic comb-like random copolymers. Colloids Surf. B Biointerfaces 146(Supplement C), 707–715 (2016)
C.R. Patra, R. Bhattacharya, E. Wang et al., Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 68(6), 1970–1978 (2008). https://doi.org/10.1158/0008-5472.CAN-1907-6102
Y. Fang, F. Du, Y. Xu et al., Enhanced cellular uptake and intracellular drug controlled release of VESylated gemcitabine prodrug nanocapsules. Colloids Surf. B Biointerfaces 128(Supplement C), 357–362 (2015)
S. Bersani, M. Vila-Caballer, C. Brazzale, M. Barattin, S. Salmaso, pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine) decorated liposomes for the delivery of gemcitabine to cancer cells. Eur J Pharm Biopharm 88(3), 670–682 (2014). https://doi.org/10.1016/j.ejpb.2014.1008.1005
S. Arpicco, C. Lerda, E. Dalla Pozza et al., Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur J Pharm Biopharm. 85(3 Pt A), 373–380 (2013). https://doi.org/10.1016/j.ejpb.2013.1006.1003
L.S. Huang, C.X. Wang, Z.L. Chen, J. Wan, X.Q. Yan, G. Duan, Preparation of gemcitabine polybutylcyanoacrylate nanoparticles. Nan Fang Yi Ke Da Xue Xue Bao. 27(11), 1653–1656 (2007)
P. Horcajada, T. Chalati, C. Serre et al., Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 9(2), 172–178 (2010). https://doi.org/10.1038/nmat2608
D. Laha, K. Pal, A.R. Chowdhuri et al., Fabrication of curcumin-loaded folic acid-tagged metal organic framework for triple negative breast cancer therapy in in vitro and in vivo systems. New J. Chem. 43(1), 217–229 (2019)
Q. Gao, J. Xu, X.-H. Bu, Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 378, 17–31 (2019)
J.-D. Xiao, H.-L. Jiang, Metal–organic frameworks for photocatalysis and photothermal catalysis. Acc. Chem. Res. 52(2), 356–366 (2019)
R.C. Huxford, J. Della Rocca, W. Lin, Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 14(2), 262–268 (2010)
M. Giménez-Marqués, T. Hidalgo, C. Serre, P. Horcajada, Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 307, 342–360 (2016)
G. Férey, C. Mellot-Draznieks, C. Serre et al., A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309(5743), 2040–2042 (2005)
V. Agostoni, P. Horcajada, M. Noiray et al., A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 5, 7925 (2015)
M.R. Di Nunzio, V. Agostoni, B. Cohen, R. Gref, A. Douhal, A “ship in a bottle” strategy to load a hydrophilic anticancer drug in porous metal organic framework nanoparticles: efficient encapsulation, matrix stabilization, and photodelivery. J. Med. Chem. 57(2), 411–420 (2014)
V. Rodriguez-Ruiz, A. Maksimenko, R. Anand et al., Efficient “green” encapsulation of a highly hydrophilic anticancer drug in metal-organic framework nanoparticles. J. Drug. Target. 23(7–8), 759–767 (2015)
W.J. Trickler, J. Khurana, A.A. Nagvekar, A.K. Dash, Chitosan and glyceryl monooleate nanostructures containing gemcitabine: potential delivery system for pancreatic cancer treatment. AAPS PharmSciTech. 11(1), 392–401 (2010). https://doi.org/10.1208/s12249-12010-19393-12240
V. Agostoni, R. Anand, S. Monti et al., Impact of phosphorylation on the encapsulation of nucleoside analogues within porous iron(iii) metal–organic framework MIL-100(Fe) nanoparticles. J. Mater. Chem. B 1(34), 4231–4242 (2013)
T. Chalati, P. Horcajada, P. Couvreur et al., Porous metal organic framework nanoparticles to address the challenges related to busulfan encapsulation. Nanomedicine (Lond). 6(10), 1683–1695 (2011). https://doi.org/10.2217/nnm.1611.1669
V. Agostoni, T. Chalati, P. Horcajada et al., Towards an improved anti-HIV activity of NRTI via metal–organic frameworks nanoparticles. Adv. Healthc. Mater. 2(12), 1630–1637 (2013). https://doi.org/10.1002/adhm.201200454
P. Horcajada, C. Serre, M. Vallet-Regi, M. Sebban, F. Taulelle, G. Ferey, Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. Engl. 45(36), 5974–5978 (2006)
C. He, D. Liu, W. Lin, Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: nanoscale metal–organic frameworks and nanoscale coordination polymers. Chem. Rev. 115(19), 11079–11108 (2015)
M.M. Yallapu, M. Jaggi, S.C. Chauhan, beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces 79(1), 113–125 (2010)
R.D. Dubey, N. Alam, A. Saneja et al., Development and evaluation of folate functionalized albumin nanoparticles for targeted delivery of gemcitabine. Int. J. Pharm. 492(1), 80–91 (2015)
J.H. Beumer, J.L. Eiseman, R.A. Parise, E. Joseph, J.M. Covey, M.J. Egorin, Modulation of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. Clin. Cancer Res. 14(11), 3529–3535 (2008)
K. Kattel, G. Mondal, F. Lin, V. Kumar, R.I. Mahato, Biodistribution of self-assembling polymer–gemcitabine conjugate after systemic administration into orthotopic pancreatic tumor bearing mice. Mol. Pharm. 14(5), 1365–1372 (2017)
A. Jain, K. Thakur, P. Kush, U.K. Jain, Docetaxel loaded chitosan nanoparticles: formulation, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 69, 546–553 (2014)
N.A. Peppas, Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60(4), 110–111 (1985)
S. Mori-Iwamoto, Y. Kuramitsu, S. Ryozawa et al., A proteomic profiling of gemcitabine resistance in pancreatic cancer cell lines. Mol. Med. Rep. 1(3), 429–434 (2008)
S.L. Loke, C.A. Stein, X.H. Zhang et al., Characterization of oligonucleotide transport into living cells. Proc. Natl Acad. Sci. U.S.A. 86(10), 3474–3478 (1989)
S. Yuan, L. Feng, K. Wang et al., Stable metal-organic frameworks: design, synthesis, and applications. Adv Mater. 30(37), e1704303 (2018)
P. Brusa, M.L. Immordino, F. Rocco, L. Cattel, Antitumor activity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. Anticancer Res. 27(1A), 195–199 (2007)
P. Horcajada, C. Serre, M. Vallet-Regi, M. Sebban, F. Taulelle, G. Ferey, Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. Engl. 45(36), 5974–5978 (2006)
P. Horcajada, R. Gref, T. Baati et al., Metal–organic frameworks in biomedicine. Chem. Rev. 112(2), 1232–1268 (2012)
L.H. Reddy, P. Couvreur, Novel approaches to deliver gemcitabine to cancers. Curr. Pharm. Des. 14(11), 1124–1137 (2008)
M. Shen, H. Cai, X. Wang et al., Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology 23(10), 105601 (2012)
D. Chen, Q. Tang, X. Li et al., Biocompatibility of magnetic Fe(3)O(4) nanoparticles and their cytotoxic effect on MCF-7 cells. Int. J. Nanomed. 7, 4973–4982 (2012)
M.S. Ewer, S.M. Ewer, Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 12(9), 547–558 (2015)
A. Albini, G. Pennesi, F. Donatelli, R. Cammarota, S. De Flora, D.M. Noonan, Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J. Natl Cancer Inst. 102(1), 14–25 (2010)
L.H. Reddy, H. Khoury, A. Paci et al., Squalenoylation favorably modifies the in vivo pharmacokinetics and biodistribution of gemcitabine in mice. Drug Metab. Dispos 36(8), 1570–1577 (2008)
Acknowledgements
Ms. Preeti Kush, Jitender Madan, and Upendra Kumar Jain acknowledge the management of the Chandigarh group of colleges, Mohali (Punjab), India & IKG Punjab Technical University, Kapurthala (Punjab), India for providing the necessary infrastructure and laboratory facilities. Akash Deep and Parveen Kumar thank the Director, CSIR-CSIO, Chandigarh for providing the necessary infrastructure and the Department of Science and Technology, India for providing a research grant under the INSPIRE Faculty award. KHK also acknowledges the support of the R&D Center for Green Patrol Technologies through the R&D for Global Top Environmental Technologies funded by the Ministry of Environment (Grant No: 2018001850001) as well as a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (Grant No. 2016R1E1A1A01940995).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kush, P., Bajaj, T., Kaur, M. et al. Biodistribution and Pharmacokinetic Study of Gemcitabine Hydrochloride Loaded Biocompatible Iron-Based Metal Organic Framework. J Inorg Organomet Polym 30, 2827–2841 (2020). https://doi.org/10.1007/s10904-019-01417-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01417-4