Abstract
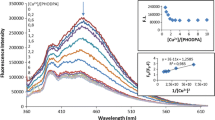
The objective of the research described herein is to report the applicability of simple Schiff base complexes, [M(L)], where M = Ni(II), Cu(II); L = N,N′-ethylenebis(acetylacetoniminate), as sensing materials and indicators in qualitative, spectroscopic and volumetric determinations of acids and bases. In this regard, UV–Vis spectroscopic properties of [M(L)] in the presence of different acids (−0.25 < pKa < 5) were investigated, and acid–base titrations using these complexes as indicators were carried out. Alcoholic solutions of [Cu(L)] and [Ni(L)] are purple and orange-red, respectively, and both solutions become decolourized upon adding strong acids (pKa < 2). During this process, the intensity of the original d–d band (λmax ~ 550 nm) decreases, and new anion-dependent CT (λmax > 700 nm) and anion-independent CT (λmax ~ 310 nm) bands, accompanied by three isobestic points, emerge. Addition of NaOH to the same solution, reproduces both the original colours and UV–Vis spectra, implying that the acid–base chemistry of [M(L)] is reversible. No such colour change occurs upon adding weak acids (pKa > 4), hence [M(L)] can be used as sensing materials for distinguishing strong acids from weak acids. In strong acid-weak base titrations, [M(L)] complexes are better indicators than methyl orange, as they give very sharp and clearly visible colour changes at the end point. The present paper reports, for the first time, the applicability of N,N′-ethylenebis(acetylacetoneiminato) nickel (II) and copper (II) Schiff base complexes as acid–base indicators in strong acid-weak base titrations where the pH at the equivalence point is ~4.












Similar content being viewed by others
References
M.Y. Udugala-Ganehenege, N.M. Dissanayake, Y. Liu, A.M. Bond, J. Zhang, Transition Met. Chem. 39, 819–830 (2014). doi:10.1007/s11243-014-9864-3
F. Denat, Y.A. Diaz-Fernandez, P. Pallavicini, L. Pasotti, Y. Rousselin, N. Sok, Dalton. Trans. 34, 6751–6758 (2009)
M. Boiocchi, L. Fabbrizzi, M. Garolfi, M. Licchelli, L. Mosca, C. Zanini, Chem. Eur. J. 15, 11288–11297 (2009)
C.M.G. dos Santos, A.J. Harte, S.J. Quinn, T. Gunnlaugsson, Coord. Chem. Rev. 252, 2512–2527 (2008)
X. Yin, J.R. Moss, Coord. Chem. Rev. 181, 27–59 (1999)
E. Fujita, D.J. Szalda, C. Creutz, N. Sutin, J. Am. Chem. Soc. 110, 4870–4871 (1988)
E. Fujita, C. Creutz, N. Sutin, D.J. Szalda, J. Am. Chem. Soc. 113, 343–353 (1991)
D.C. Olson, J. Vasilevskis, Inorg. Chem. 10, 463–470 (1971)
K. Mochizuki, S. Manaka, I. Takeda, T. Kondo, Inorg. Chem. 35, 5132–5136 (1996)
K.Y. Russ, Chem. Rev. 59, 1150–1156 (1990)
J. Schneider, H. Jia, K. Kobiro, D.E. Cabelli, J.T. Muckerman, E. Fujita, Energy Environ. Sci. 5, 9502–9510 (2012)
B.J. Fisher, R. Eisenberg, J. Am. Chem. Soc. 102, 7361–7363 (1980)
R.A. Kalil, A.H. Jalil, A.Y. Abd-Alrazzak, J. Iran. Chem. Soc. 6, 345–352 (2009)
V.G. Makhankova, O.Y. Vassilyeva, V.N. Kokozay, B.W. Skelton, J. Reedijk, G.A. Vanalbada, L. Sorace, D. Gatteschi, New J. Chem. 25, 685 (2001)
S.K. Gupta, P.B. Hitchcock, Y.S. Kushwah, J. Coord. Chem. 55, 1401–1407 (2002)
G.R. Clark, D. Hail, T.N. Waters, Inorg. Phys. Theor. J. Chem. Soc. (A), 223–226 (1968) http://pubs.rsc.org| doi:10.1039/J19680000223
S.K. Kang, H.S. Kim, Y.N. Kim, Bull. Korean Chem. Soc. 25(12), 1959–1960 (2004)
M.Y. Udugala-Ganehenege, M.J. Heeg, L.M. Hryhorczuk, L.E. Wenger, J.F. Endicott, Inorg. Chem. 40, 1614–1625 (2001)
J.G. Martin, S.C. Cummings, Inorg. Chem. 12, 1477–1482 (1973)
Acknowledgments
The authors gratefully acknowledge financial assistance from National Research Foundation of Sri Lanka through the research Grant RG/2006/FR/02. The authors thank Prof. J. F. Endicott of Wayne State University, Detroit, MI 48202, USA and Prof. O.A. Ileperuma of University of Peradeniya, Sri Lanka for reviewing the manuscript before submission. The comments given by Prof. Alan Bond and Dr. Jie Zhang of Monash University are also gratefully acknowledged. The assistance given by Ms. M. C. R. Peiris, Ms. T. Navarathne, Mr. Dinesh Nugegoda and Mr. Dilan Karunathilaka, for repeating the spectroscopic experiments and carrying out titrations, is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Udugala-Ganehenege, M.Y., Dissanayake, N.M. & Adhikari, A.M.K.S.P. Application of N,N′-ethylenebis(acetylacetoneiminato) Nickel (II) and Copper (II) Schiff Base Complexes as Acid–Base Sensing Materials and Indicators in Volumetric Titrations : Qualitative, Spectroscopic and Titrimetric Analyses. J Inorg Organomet Polym 25, 964–974 (2015). https://doi.org/10.1007/s10904-015-0200-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0200-y