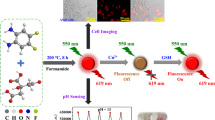
Abstract
Fluorescent carbon dots (Trp-CDs) were prepared using tryptophan as precursor and were characterized on the basis of elemental analysis, powder-XRD, IR, Raman spectroscopy, 13C-NMR, UV–Vis, fluorescence and TEM. Trp-CDs exhibit poor fluorescence in 100% water but showed strong Aggregation Induced Emission (AIE) in ethanol and higher alcohols. The anion sensing study of Trp-CD revealed that it selectively detects CN− and Cr2O7−2 and from fluorescence quenching titration study, quenching constant, LOD and range of detection were evaluated. The emission life-time of Trp-CD before and after addition of CN− and Cr2O7−2 were measured, the decay curve before addition of anion was best fitted with a bi-exponential function with life-time of τ1 2.79 ns (10.74%) and τ2 18.93 ns (89.26%). The mechanistic study revealed that for CN−, the fluorescence quenching is due to its interaction with protons attached to surface functional groups and for Cr2O7−2, it is due to inner filter effect (IFE). Sensing strips were prepared by coating Trp-CDs onto various solid surfaces including agarose films and were used for detection of CN− and Cr2O7−. Trp-CD was found to be nontoxic and biocompatible and used as staining agent for Artemia and Bacteria (Bacillus Subtilis, Pseudomonas) and detection of CN− and Cr2O7−.
Graphical Abstract













Similar content being viewed by others
Availability of Data and Materials
The online version of this article (https://doi.org/…….) contains supplementary material, which is available to authorized users.
References
Rajamanikandan R, Sasikumar K, Kosame S, Ju H (2023) Optical sensing of toxic cyanide anions using noble metal. Nanomaterials 13:290
Zhu M, Sun L, Liu X, Pang X, Fan F, Yang X, Hua R, Wang Y (2023) A reversible CHEF-based NIR fluorescent probe for sensing Hg2+ and its multiple application in environmental media and biological systems. Sci Total Env 874:162460
Kongsanan N, Pimsin N, Keawprom C, Sricharoen P, Areerob Y, Nuengmatcha P, Oh W-C, Saksit C (2021) Limchoowong N (2021) A fluorescence switching sensor for sensitive and selective detections of cyanide and ferricyanide using mercuric cation-graphene quantum dots. ACS Omega 6:14379–14393
Zhu M, Zhao Z, Liu X, Chen P, Fan F, Wu X, Hua R, Wang Y (2021) A novel near-infrared fluorimetric method for point-of-care monitoring of Fe2+ and its application in bioimaging. J Hazard Mat 406:124767
Ji C, Zhou Y, Leblanc RM, Peng Z (2020) Recent developments of carbon dots in biosensing: a review. ACS Sens 5:2724–2741
Sharma A, Das J (2019) Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging, and biomedicine. Nanobiotechnol 17:Article No 92
Sharma V, Singh SK, Mobin SM (2019) Bioinspired carbon dots: from rose petals to tunable emissive nanodots. Nanoscale Adv 1:1290–1296
Bhatt M, Bhatt S, Vyas G, Raval IH, Haldar S, Paul P (2020) Water-dispersible fluorescent carbon dots as bioimaging agents and probes for Hg2+ and Cu2+ ions. ACS Appl Nano Mater 3:7096–7104
Li P, Li SFY (2020) Recent advances in fluorescence probes based on carbon dots for sensing and speciation of heavy metals. Nanophotonics 10:877–908
Arshad F, Pal A, Md Palashuddin SK (2021) Review—aggregation-induced emission in carbon dots for potential applications. ECS J Solid State Sci Techno 10:021001
Juang RS, Fu CC, Hsieh CT, Gu S, Gandomi YA, Liu SH (2020) Highly luminescent aggregate-induced emission from polyethylene glycol-coated carbon quantum dot clusters under blue light illumination. J Mater Chem C 8:16569
Liu ZX, Wu ZL, Gao MX, Liu H, Huang CZ (2016) Carbon dots with aggregation induced emission enhancement for visual permittivity detection. Chem Commun 52:2063
Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ (2015) Aggregation-induced emission: together we shine, united we soar. Chem Rev 115:11718–11940
Chen Y, Lam JWY, Kwok RTK, Liu B, Tang BZ (2019) Aggregation-induced emission: fundamental understanding and future developments. Mater Horiz 6:428–433
Wurthner F (2020) Aggregation-induced emission (AIE): a historical perspective. Angew Chem Int Ed 59:14192–14196
Wu Z, Yao Q, Zang SQ, Xie J (2021) Aggregation-induced emission in luminescent metal nanoclusters. Natl Sci Rev 8:nwaa208
Wang C, Jiang K, Xu Z, Lin H, Zhang C (2016) Glutathione modified carbon-dots: from aggregation-induced emission enhancement properties to a “turn-on” sensing of temperature/Fe3+ ions in cells. Inorg Chem Front 3:514–522
Singh VD, Singh RS, Paitandi RP, Dwivedi BK, Maiti B, Pandey DS (2018) Solvent-dependent self-Assembly and aggregation-induced emission in Zn(II) complexes containing phenothiazine-based terpyridine ligand and its efficacy in pyrophosphate sensing. J Phys Chem C 122:5178–5187
Wang F, Wang L, Chen X, Yoon J (2014) Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem Soc Rev 43:4312–4324
Xu Z, Chen X, Kim HN, Yoon J (2010) Sensors for the optical detection of cyanide ion. Chem Soc Rev 39:127–137
Kulig W (1991) Cyanide Toxicity; U.S. Department of Health and Human Services. LAND WA30 no.15 Atlanta GA 30333
Vennesland B, Comm EE, Knownles CJ, Westly J, Wissing F (1981) Cyanide in Biology. Academic Press, London
Acheampong MA, Meulepas RJW, Lens PNL (2010) Removal of heavy metals and cyanide from gold mine wastewater. J Chem Technol Biotechnol 85:590–613
Khairnar N, Tayade K, Bothra S, Sahoo SK, Singh J, Singh N, Bendre R, Kuwar A (2014) Novel fluorescent chemosensing of CN− anions with nanomolar detection using the Zn2+–isonicotinohydrazide metal complex. RSC Adv 4:41802–41806
Kumar A, Maity D, Vyas G, Bhatt M, Bhatt S, Paul P (2021) Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles for detection and absorptive removal of cyanide from aqueous media with high efficiency. Colloids Surf A: Physicochem Eng Aspects 617:126358
Bolarinwa F, Orfila C, Morgan MRA (2014) Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem 152:133–139
Dagiliene M, Martynaitis V, Krisciuniene V, Ktolaityte S, Ackus AS (2015) Colorimetric cyanide chemosensor based on 1′,3,3′,4-tetrahydrospiro[chromene-2,2′-indole]. ChemistryOpen 4:363–369
Dinker MK, Kulkarni PS (2015) Recent advances in silica-based materials for the removal of hexavalent chromium: a review. J Chem Eng Data 60:2521–2540
Rathi BS, Kumar PS, Show PL (2021) A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J Hazard Mater 409:124413–124492
Dakiky M, Khamis M, Manassra A, Mereb M (2002) Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Advan Environ Res 6:533–540
U. S. EPA (2018) Edition of the drinking water standards and health advisories tables, Office of Water, Washington DC EPA 822-F-18-001: March 2018
Hagendorfer H, Goessler W (2008) Separation of chromium(III) and chromium(VI) by ion chromatography and an inductively coupled plasma mass spectrometer as element-selective detector. Talanta 76:656–661
Desharnais B, Huppé G, Lamarche M, Mireault P, Skinner CD (2012) Cyanide quantification in post-mortem biological matrices by headspace GC-MS. Forensic Sci Int 222:346–351
Sulistyarti H, Kolev SD (2013) Online ligand exchange in the determination of weak acid dissociable cyanide by gas diffusion-flow injection analysis. Microchem J 111:103–107
Balamurugan G, Velmathi S (2016) Novel chromogenic selective sensors for aqueous cyanide ions under high water content and real sample analysis. Anal Methods 8:1705–1710
Sukato R, Sangpetch N, Palaga T, Jantra S, Vchirawongkwin V, Jongwohan C, Sukwattanasinitt M, Wacharasindhu S (2016) New turn-on fluorescent and colorimetric probe for cyanide detection based on BODIPY-salicylaldehyde and its application in cell imaging. J Hazard Mater 314:277–285
Long L, Yuan X, Cao S, Han Y, Liu W, Chen Q, Han A, Wang K (2019) Determination of cyanide in water and food samples using an efficient naphthalene-based ratiometric fluorescent probe. ACS Omega 4:10784–10790
Maity D, Vyas G, Bhatta M, Paul P (2015) Detection of NaCN in aqueous media using a calixarene-based fluoroionophore containing ruthenium(II)-bipyridine as the fluorogenic unit. RSC Adv 5:6151–6159
Nhien PQ, Chou WL, Cuc TTK, Khang TM, Wu CH, Thirumalaivasan N, Hue BTB, Wu TJI, Wu SP, Lin HC (2020) Multi-stimuli responsive FRET processes of bifluorophoric AIEgens in an amphiphilic copolymer and Its application to cyanide detection in aqueous media. ACS Appl Mater Interfaces 12:10959–10972
Oh J, Jeon I, Kim D, You Baek D, Kang SJ, Lee J (2020) Highly stable upconverting nanocrystal–polydiacetylenes nanoplates for orthogonal dual signaling-based detection of cyanide. ACS Appl Mater Interfaces 12:4934–4943
Hajizadeh S, Farhadi K, Forough M, Sabzi RE (2011) Silver nanoparticles as a cyanide colorimetric sensor in aqueous media. Anal Methods 3:2599–2603
Zeng J, Cao Y, Chen J, Wang X, Yua J, Yu B, Yan Z, Chend X (2014) Au@Ag core/shell nanoparticles as colorimetric probes for cyanide sensing. Nanoscale 6:9939–9943
Bhatt S, Vyas G, Paul P (2022) Rosmarinic acid-capped silver nanoparticles for colorimetric detection of CN– and redox-modulated surface reaction-aided detection of Cr(VI) in water. ACS Omega 7:1318–1328
Kumar A, Maity D, Vyas G, Bhatt M, Bhatt S, Paul P (2021) Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles for detection and absorptive removal of cyanide from aqueous media with high efficiency. Colloids Surf A: Physico Eng Aspects 617:126358
Ensafi AA, Kazemifard N, Rezaei B (2015) Label-free and turn-on fluorescent cyanide sensor based on CdTe quantum dots using silver nanoparticles. RSC Adv 5:40088–40093
Molaei MJ (2020) Principles, mechanisms, and application of carbon quantum dots in sensors: a review. Anal Methods 12:1266–1287
Bhatt S, Vyas G, Paul P (2022) Microwave-assisted synthesis of nitrogen-doped carbon dots using prickly pear as the carbon source and its application as a highly selective sensor for Cr(VI) and as a patterning agent. Anal Methods 14:269–277
Bhatt S, Bhatt M, Kumar A, Vyas G, Gajaria T, Paul P (2018) Green route for synthesis of multifunctional fluorescent carbon dots from Tulsi leaves and its application as Cr(VI) sensors, bio-imaging and patterning agents. Colloids Surf, B 167:126–133
Grabolle M, Spieles M, Lesnyak V, Gaponik N, Eychmuller A, Genger UR (2009) Determination of the fluorescence quantum yield of quantum dots: suitable procedures and achievable uncertainties. Anal Chem 81:6285–6294
Kwon W, Do S, Lee J, Hwang S, Kim JK, Rhee SW (2013) Freestanding luminescent films of nitrogen-rich carbon nanodots toward large-scale phosphor-based white-light-emitting devices. Chem Mater 25:1893–1899
Peng H, Sejdic JT (2009) Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem Mater 21:5563–5565
Ding H, Yu SB, Xiong WJS, HM, (2016) Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10:484–491
Fileti EE, Chaudhuri P, Canuto S (2004) Relative strength of hydrogen bond interaction in alcohol–water complexes. Chem Phys Lett 400:494–499
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem 125:4045–4049
Li W, Zhang Z, Kong B, Feng S, Wang J, Wang L, Yang J, Zhang F, Wu P, Zhao D (2013) Simple and green synthesis of nitrogen-doped photoluminescent carbonaceous nanospheres for bioimaging. Angew Chem 52:8151–8155
Qian Z, Ma J, Shan X, Feng H, Shao L, Chen J (2014) Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem Eur J 20:2254–2263
Chen Y, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132–5138
Jia X, Li J, Wang E (2012) One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 4:5572–5575
Qu S, Chen H, Zheng X, Cao J, Liu X (2013) Ratiometric fluorescent nanosensor based on water soluble carbon nanodots with multiple sensing capacities. Nanoscale 5:5514–5518
Acknowledgements
CSIR-CSMCRI Registration No. is 217/2021. P. P. Gratefully acknowledges CSIR for the financial support in the form of CSIR-Emeritus Scientist Scheme (CSIR-ES Scheme No. 21(1046)/18/EMR-II). S. Bhatt (CSIR Award No.: 31/28(0240)/2018-EMR-I) and G. Vyas (CSIR- Award No.: 31/28(0238)/2018-EMR-I) acknowledge CSIR for awarding the Senior Research Fellowship. A. Kumar (Award No. 19-06/2011(i)EU-IV) gratefully acknowledges UGC for awarding the Research Fellowship (JRF and SRF). All the authors acknowledge CSIR-CSMCRI for providing research facilities and partial research expense related to this work. We thank Dr. G. R. Bhadu, for recording TEM and Laiya Riddhi P. for XRD, V. Agrawal for recording IR spectra, V. Bakani for elemental analysis and Raman spectra. We thank AESD&CIF for additional analytical support.
Funding
Details of funding received is incorporated in the Acknowledgement.
Author information
Authors and Affiliations
Contributions
M.B. Conceptualization of work, methodology and wrote draft of the manuscript. S.B. Data curation and formal analysis. G.V. Data analysis and validation. I.R. Toxicity study, imaging and prepared Figures 11 and 12. A.K. Application study with sensing strips. P.P. Conceptualization, funding acquisition, supervision and writing – review and editing of manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatt, M., Bhatt, S., Vyas, G. et al. Fluorescent Carbon Dots: Aggregation-Induced Emission Enhancement, Application as Probe for CN− and Cr2O7−2, Sensing Strips and Bio-imaging Agent. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03602-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03602-2