Abstract
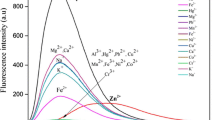
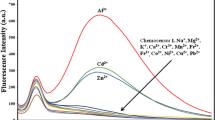
We designed a novel carbazole-based chemosensor from 2-(N-hexylcarbazol-3’-yl)-pyridine-5-carbaldehyde which was named probe 7b. The main purpose of this study is to investigate whether metal ions in liquid media can be detected with probe 7b. The details were presented in this paper. First, the molecular absorption and fluorescence properties of probe 7b were characterized by spectrophotometers. Then, several methods were applied to check its sensing properties. The results showed that probe 7b has a sense towards Fe3+ ion than other interfering metal ions. The selectivity and sensitivity of probe 7b towards Fe3+ were very satisfactory to use in applications. Also, it was observed that when aqueous Fe3+ ion solutions were added to probe 7b in dimethyl sulfoxide (DMSO), the fluorescence intensity of probe 7b decreased. This situation (turn-off of emission) is due to the paramagnetic effect between probe 7b and Fe3+ ions. The limit of detection (LOD) value was found as 1.38 nM for probe 7b. This value is very small to compete with its counterparts in the literature. A real sample experiment indicated that probe 7b can detect Fe3+ ions more than other ions in real media, too. As a result, it was deduced that probe 7b is a very strong candidate to use in sensor technology.












Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Kandemir H, Kocak A, Tumay SO, Cosut B, Zorlu Y, Sengul IF (2018) Experimental and theoretical studies of carbazole-based Schiff base as a fluorescent Fe3 + probe.Turk J Chem.42
Yang L, Zhu W, Fang M, Zhang Q, Li C (2013) A new carbazole-based Schiff-base as fluorescent chemosensor for selective detection of Fe3 + and Cu2+. Spectrochim Acta A Mol Biomol Spectrosc 109:186–192
Chen J-F, Cheng X-B, Li H, Lin Q, Yao H, Zhang Y-M, Wei T-B (2017) A copillar[5]arene-based fluorescence “on–off–on” sensor is applied in sequential recognition of an iron cation and a fluoride anion. New J Chem 41:2148–2153
Tümay SO, Sarıkaya SY, Yeşilot S (2018) Novel iron(III) selective fluorescent probe based on synergistic effect of pyrene-triazole units on a cyclotriphosphazene scaffold and its utility in real samples. J Luminescence 196:126–135
Pepper SE, Borkowski M, Richmann MK, Reed DT (2010) Determination of ferrous and ferric iron in aqueous biological solutions. Anal Chim Acta 663:172–177
Pundi A, Chang C-J, Chen J, Hsieh S-R, Lee M-C (2021) A chiral carbazole based sensor for sequential “on-off-on” fluorescence detection of Fe3 + and tryptophan/histidine.Sensors and Actuators B: Chemical.328
He W, Liu Z (2016) A fluorescent sensor for Cu2 + and Fe3 + based on multiple mechanisms. RSC Adv 6:59073–59080
Bayraktutan T, Gur B, Onganer Y (2022) A new FRET-based functional chemosensor for fluorometric detection of Fe3 + and its validation through in silico studies.Journal of Molecular Structure.1256
Şenol AM, Onganer Y (2022) A novel “turn-off” fluorescent sensor based on cranberry derived carbon dots to detect iron (III) and hypochlorite ions.Journal of Photochemistry and Photobiology A: Chemistry.424
Bozkurt E, Arık M, Onganer Y (2015) A novel system for Fe3 + ion detection based on fluorescence resonance energy transfer. Sens Actuators B: Chem 221:136–147
Danjou P-E, Lyskawa J, Delattre F, Becuwe M, Woisel P, Ruellan S, Fourmentin S, Cazier-Dennin F (2012) New fluorescent and electropolymerizable N-azacrown carbazole as a selective probe for iron (III) in aqueous media. Sens Actuators B: Chem 171–172:1022–1028
Aslandaş AM, Balcı N, Arık M, Şakiroğlu H, Onganer Y, Meral K (2015) Liquid nitrogen-assisted synthesis of fluorescent carbon dots from Blueberry and their performance in Fe3 + detection. Appl Surf Sci 356:747–752
Zhu W, Yang L, Fang M, Wu Z, Zhang Q, Yin F, Huang Q, Li C (2015) New carbazole-based Schiff base: colorimetric chemosensor for Fe3 + and fluorescent turn-on chemosensor for Fe3 + and Cr3+. J Luminescence 158:38–43
Şenol AM, Onganer Y, Meral K (2017) An unusual “off-on” fluorescence sensor for iron(III) detection based on fluorescein–reduced graphene oxide functionalized with polyethyleneimine. Sens Actuators B: Chem 239:343–351
Nelson M, Muniyasamy H, Kubendran AM, Balasubramaniem A, Sepperumal M, Ayyanar S (2021) Carbazole based fluorescent chemosensor for the meticulous detection of tryptamine in aqueous medium and its efficacy in cell-imaging and molecular logic gate. J Mol Liq 337:116445
Tang L, Wu D, Wen X, Dai X, Zhong K (2014) A novel carbazole-based ratiometric fluorescent sensor for Zn2 + recognition through excimer formation and application of the resultant complex for colorimetric recognition of oxalate through IDAs. Tetrahedron 70:9118–9124
Christopher Leslee DB, Karuppannan S, Vengaian KM, Gandhi S, Subramanian S (2017) Carbazole–azine based fluorescence ‘off–on’ sensor for selective detection of Cu2 + and its live cell imaging. Luminescence 32:1354–1360
Chao J, Duan Y, Zhang Y, Huo F, Yin C, Xu M, Li M (2020) A carbazole-based fluorescent probe for ultra-fast detection of ClO – and its application to live cell imaging. Chem Papers 74:1171–1176
Ho C-L, Wong W-Y, Gao Z-Q, Chen C-H, Cheah K-W, Yao B, Xie Z-Y, Wang Q, Ma D-G, Wang L-X, Yu X-M, Kwok H-S, Lin Z-Y (2008) Red-Light-Emitting Iridium Complexes with hole-transporting 9-Arylcarbazole moieties for Electrophosphorescence Efficiency/Color Purity Trade-off optimization. Adv Funct Mater 18:319–331
Xu B, Sheibani E, Liu P, Zhang J, Tian H, Vlachopoulos N, Boschloo G, Kloo L, Hagfeldt A, Sun L (2014) Carbazole-based hole-transport materials for efficient solid-state dye-sensitized solar cells and Perovskite Solar cells. Adv Mater 26:6629–6634
Nepomnyashchii AB, Parkinson BA (2014) Contrasting electrogenerated chemiluminescence for a dissolved and surface-attached carbazole thiophene cyanoacrylate dye. ACS Appl Mater Interfaces 6:14881–14885
Altinolcek N, Battal A, Tavasli M, Peveler WJ, Yu HA, Skabara PJ (2020) Synthesis of novel multifunctional carbazole-based molecules and their thermal, electrochemical and optical properties. Beilstein J Org Chem 16:1066–1074
Misra R, Mandal A, Mukhopadhyay M, Maity DK, Bhattacharyya SP (2009) Spectral signatures of intramolecular charge transfer process in β-Enaminones: a combined experimental and theoretical analysis. J Phys Chem B 113:10779–10791
Liu L, Liu X, Guo C, Fang M, Li C, Zhu W (2021) Carbazole-based dual-functional chemosensor: colorimetric sensor for Co2 + and fluorescent sensor for Cu2 + and its application. J Chin Chem Soc 68:2368–2377
Zhao H-W, Wu G, Sun X-Y, Chao J-B, Li Y, Jiang L, Han H (2018) A highly selective and ratiometric molecular probe for cyanide sensing based on a phenothiazine-hemicyanine dye. J Luminescence 201:474–478
Karuk Elmas SN, Gunay IB, Genc HN, Aydin D, Arslan FN, Sadi G, Sirit A, Yilmaz I (2020) A tetraoxacalix[2]arene[2]triazine based fluorogenic probe for the sensing of Fe3+: computational and living–cell imaging applications.Journal of Photochemistry and Photobiology A: Chemistry.403
Zhang Y, Wang G, Zhang J (2014) Study on a highly selective fluorescent chemosensor for Fe3 + based on 1,3,4-oxadiazole and phosphonic acid. Sens Actuators B: Chem 200:259–268
Bao X, Cao X, Nie X, Xu Y, Guo W, Zhou B, Zhang L, Liao H, Pang T (2015) A new selective fluorescent chemical sensor for Fe3 + based on rhodamine B and a 1,4,7,10-tetraoxa-13-azacyclopentadecane conjugate and its imaging in living cells. Sens Actuators B: Chem 208:54–66
Zhang Z, Li F, He C, Ma H, Feng Y, Zhang Y, Zhang M (2018) Novel Fe3 + fluorescence probe based on the charge-transfer (CT) molecules. Sens Actuators B: Chem 255:1878–1883
Şenkuytu E, Eçik ET, Çoşut B (2018) Bodipy decorated triazine chemosensors for ag + ions with high selectivity and sensitivity. J Luminescence 203:639–645
Immanuel David C, Prabakaran G, Karuppasamy A, Veetil JC, Kumar RS, Almansour AI, Perumal K, Ramalingan C, Nandhakumar R (2022) A single carbazole based chemosensor for multiple targets: sensing of Fe3 + and arginine by fluorimetry and its applications. J Photochem Photobiol A 425:113693
Yang M, Sun M, Zhang Z, Wang S (2013) A novel dansyl-based fluorescent probe for highly selective detection of ferric ions. Talanta 105:34–39
Patil SK, Das D (2020) A novel rhodamine-based optical probe for mercury(II) ion in aqueous medium: a nanomolar detection, wide pH range and real water sample application. Spectrochim Acta Part A Mol Biomol Spectrosc 225:117504
Acknowledgements
The authors are grateful to Uludağ University and Atatürk University for the support of this work.
Funding
This work was supported by Scientific Research Programme (BAP) from Uludağ University [Project #: KUAP (F)-2018/14] and Atatürk University [Project #: FBA-2021-8808].
Author information
Authors and Affiliations
Contributions
Investigation, Validation, Visualization: [Ahmet Battal], Investigation, Validation, Visualization: [Solomon Bezabeh Kassab], Investigation, Validation, Visualization: [Nuray Altinolcek Gultekin], Conceptualization, Writing - original draft, Supervision: [Mustafa Tavasli], Conceptualization, Writing - original draft, Supervision: [Yavuz Onganer].
Corresponding authors
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battal, A., Kassa, S.B., Gultekin, N.A. et al. A Carbazole-based Fluorescent Turn-off Chemosensor for Iron (II/III) Detection in a Dimethyl Sulfoxide. J Fluoresc 33, 1421–1429 (2023). https://doi.org/10.1007/s10895-023-03156-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03156-9