Abstract
A new fluorescence probe (L) selectively detecting Al3+ ions was synthesized via the condensation reaction, and characterized using UV–Vis, FT-IR, 1H-NMR and 13C-NMR spectroscopic techniques. The limit of detection for Al3+ ions of this synthesized probe was found to be 9.29 × 10–7 M, while the Ka constant value was determined to be 1.64 × 104 M−1. The stoichiometric binding ratio of L-Al3+ was found to be 2:1 using the Job’s plot method, and this ratio was also confirmed by 1H-NMR titration and mass spectrometry. The recyclability of the chemosensor was found by the fluorescence method through the addition of EDTA to the L-Al3+ solution. The obtained data showed that the carbazole-based Schiff base acted as an ideal chemosensor for Al3+.
Graphical Abstract
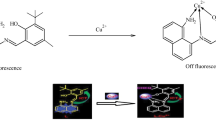
Carbazole-based Schiff base as a fluorescent sensor for detection of Al3+ was synthesized and characterized. The association constant (Ka) was calculated to be 1.64 × 104 M−1 and the limit of detection (LOD) value was determined to be 9.29 × 10–7 M. It was determined that th Schiff base was bound to Al3+ ions in 2:1 stoichiometric ratio. In the presence of other competitive metal cations, the selectivity of sensor L to Al3+ was not significantly affected.











Similar content being viewed by others
Availability of Data and Material
No access connection.
References
Burgess J (1996) Man and the elements of group 3 and 13. Chem Soc Rev 25:85–92
Srinivasan P, Viraraghavan T, Subramanian K (1999) Aluminium in drinking water: An Overview. Water SA 25:47–55
Minshall C, Nadal J, Exley C (2014) Aluminium in human sweat. J Trace Elem Med Biol 28:87–88
(2008) Guidelines for drinking water quality incorporating 1st and 2nd addenda recommendations, 3rd edn. World Health Organization, Geneva, Switzerland
Perl DP, Brody AR (1980) Alzheimer’s disease: X-Ray Spectrometric Evidence of Aluminum Accumulation in Neurofibrillary Tangle-Bearing Neurons. Science 208:297–299
Perl D, Gajdusek D, Garruto R, Yanagihara R, Science GC (1982) Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and parkinsonism-dementia of guam. Science 217:1053–1055
Poot-Poot W, Hernandez-Sotomayor SM (2011) Aluminum stress and its role in the phospholipid signaling pathway in plants and possible biotechnological applications. IUBMB Life 63:864–872
Cronan C, Walker W, Bloom PN (1986) Predicting aqueous aluminium concentrations in natural waters. Nature 324:140–143
Polizzi S, Pira E, Ferrara M, Bugiani M, Papaleo A, Albera R, Palmi S (2002) Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology 23:761–764
Yokel RA (2002) Aluminum chelation principles and recent advances. Coord Chem Rev 228:97–113
Cannon JR, Greenamyre JT (2011) The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124:225–250
Upadhyay KK, Kumar A (2010) Pyrimidine based highly sensitive fluorescent receptor for Al3+ showing dual signalling mechanism. Org Biomol Chem 8:4892–4897
Sahana A, Banerjee A, Lohar S, Sarkar B, Mukhopadhyay SK, Das D (2013) Rhodamine-based fluorescent probe for al3+ through time-dependent PET–CHEF–FRET processes and ıts cell staining application. Inorg Chem 52:3627–3633
Das S, Sahana A, Banerjee A, Lohar S, Safin DA, Babashkina M, Bolte M, Garcia Y, Hauli I, Mukhopadhyay SK, Das D (2013) Ratiometric fluorescence sensing and intracellular imaging of Al3+ ions driven by an intramolecular excimer formation of a pyrimidine–pyrene scaffold. Dalton Trans 42:4757–4763
Kim S, Noh JY, Kim KY, Kang HK, Nam SW, Kim SH, Park S, Kim C, Kim J (2012) Salicylimine-based fluorescent chemosensor for aluminum ıons and application to bioimaging. Inorg Chem 51:3597–3602
Shellaiah M, Wu YH, Lin HC (2013) Simple pyridyl-salicylimine-based fluorescence “turn-on” sensors for distinct detections of Zn2+, Al3+ and OH− ions in mixed aqueous media. Analyst 138:2931–2942
Bansod B, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455
Gong T, Liu J, Liu X, Liu J, Xiang J, Wu Y (2016) A sensitive and selective sensing platform based on CdTe QDs in the presence of l-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem 213:306–312
Duong TQ, Kim JS (2010) Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem Rev 110:6280–6301
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244
Berhanu AL, Gaurav MI, Malik AK, Aulakh JS, Kumar V, Kim KH (2019) A review of the applications of Schiff bases as optical chemical sensors. Trend Anal Chem 116:74–91
Abu-Dief AM, Mohamed IM (2015) A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ J Basic Appl Sci 4:119–133
Yeldir EK, Erdener D, Kaya İ (2022) Synthesis and characterization of a pyrene-based Schiff base and its oligomer: Investigation of fluorescent Cr3+ probe. React Funct Polym 170:105097
(a) Udhayakumari D, Inbaraj V (2020) A review on Schiff base fluorescent chemosensors for cell imaging applications. J Fluoresc 30:1203–1223. (b) Abdel-Rahman LH, Abu-Dief A, Abdel-Mawgoud A (2019) Design, synthesis and structural inspection of some novel di- and tri-azomethene compounds as chemo sensors for the detection of various metal ions. Ann Chem Sci Res 1:1–16
Zhu WJ, Wu ZY, Song JM, Li C (2011) Preparation and properties of two new soluble carbazole-containing functional polyacetylenes. Chin Chem Lett 22:1017–1020
Wang HY, Chen G, Xu XP, Chen H, Ji SJ (2010) The synthesis and optical properties of benzothiazole-based derivatives with various π-electron donors as novel bipolar fluorescent compounds. Dyes Pigments 86:238–248
Chuang CN, Chuang HJ, Wang YX, Chen SH, Huang JJ, Leung M, Hsieh KH (2012) Polymers with alkyl main chain pendent biphenyl carbazole or triphenylamine unit as host for polymer light emitting diodes. Polymer 53:4983–4992
Yoon KR, Ko SO, Lee H, Lee SM (2007) Synthesis and characterization of carbazole derived nonlinear optical dyes. Dyes Pigments 75:567–573
Tsai YL, Chang CC, Kang CC, Chang TC (2007) Effect of different electronic properties on 9-aryl-substituted BMVC derivatives for new fluorescence probes. J Lumin 127:41–47
Zhu W, Yang L, Fang M, Wu Z, Zhang Q, Yin F, Huang Q, Li C (2015) New carbazole-based Schiff base: Colorimetric chemosensor for Fe3+ and fluorescent turn-on chemosensor for Fe3+ and Cr3+. J Lumin 158:38–43
Hsiao S-H, Chen Y-Z (2018) Electrosynthesis of redox-active and electrochromic polymer films from triphenylamine-cored star-shaped molecules end-capped with arylamine groups. Eur Polymer J 99:422–436
Schaming D, Costa Coquelard C, Lampre I, Sorgues S, Erard M, Liu X, Liu J, Sun L, Canny J, Thouvenot R, Ruhlmann L (2010) Formation of a new hybrid complex via coordination interaction between 5,10,15-tritolyl-20-(4- and 3-pyridyl)porphyrin or 5,10,15-triphenyl-20-(4-pyridyl)porphyrin and the α-[MSiW11O39]6− Keggin-type polyoxometalate (M = Co2+ and Ni2+). Inorg Chim Acta 363:2185–2192
Kolcu F, Erdener D, Kaya İ (2020) A Schiff base based on triphenylamine and thiophene moieties as a fluorescent sensor for Cr (III) ions: Synthesis, characterization and fluorescent applications. Inorg Chim Acta 509:119676
Bekhradnia A, Domehri E, Khosravi M (2016) Novel coumarin-based fluorescent probe for selective detection of Cu(II). Spectrochim Acta A Mol Biomol Spectrosc 152:18–22
Zhang C, Gao B, Zhang Q, Zhang G, Shuang S, Dong C (2016) A simple Schiff base fluorescence probe for highly sensitive and selective detection of Hg2+and Cu2+. Talanta 154:278–283
Shi Z, Zhao Z (2019) Microwave irradiation synthesis of novel indole triazole Schiff base fluorescent probe for Al3+ ion. Inorg Chim Acta 498:119135
Kolcu F, Erdener D, Kaya İ (2021) Synthesis and characterization of a highly selective turn-on fluorescent chemosensor for Sn2+ derived from diimine Schiff base. Synth Met 272:116668
Al- Abdulkarim HA, El-khatib RM, Aljohani FS, Mahran A, Alharbi A, Mersal GAM, El-Metwaly NM, Abu-Dief AM (2021) Optimization for synthesized quinoline-based Cr3+, VO2+, Zn2+ and Pd2+ complexes: DNA interaction, biological assay and in-silico treatments for verification. J Mol Liq 339:116797
Zeng S, Li SJ, Sun XJ, Li MQ, Xing ZY, Li JL (2019) A benzothiazole-based chemosensor for significant fluorescent turn-on and ratiometric detection of Al3+ and its application in cell imaging. Inorg Chim Acta 486:654–662
Kundu BK, Mandal P, Mukhopadyay BG, Tiwari R, Nayak D, Ganguly R, Mukhopadhay S (2019) Substituent dependent sensing behavior of Schiff base chemosensors in detecting Zn2+and Al3+ ions: Drug sample analysis and living cell imaging. Sens Actuators B Chem 282:347–358
Yoon J, Czarnik AW (1992) Fluorescent chemosensors of carbohydrates. A means of chemically communicating the binding of polyols in water based on chelation-enhanced quenching. J Am Chem Soc 114:5874–5875
Tsui YK, Devaraj S, Yen YP (2012) Azo dyes featuring with nitrobenzoxadiazole (NBD) unit: A new selective chromogenic and fluorogenic sensor for cyanide ion. Sens Actuat B: Chem 161:510–519
Kolcu F, Kaya İ (2022) Carbazole-based Schiff base: A sensitive fluorescent ‘turn-on’ chemosensor for recognition of Al(III) ions in aqueous-alcohol media. Arab J Chem 15:103935
Zhang Y, Fang Y, Xu N, Zhang MQ, Wu GZ, Yao C (2016) A colorimetric and ratiometric fluorescent chemosensor based on furan-pyrene for selective and sensitive sensing Al3+. Chin Chem Lett 27:1673–1678
Kaur N, Kaur B (2017) Spectral studies on anthracene based dual sensor for Hg2+ and Al3+ ions with two distinct output modes of detection. Spectrochim Acta Part A Mol Biomol Spectrosc 181:60–64
Kumar J, Sarma M, Phukan P, Das DK (2015) A new simple Schiff base fluorescence “on” sensor for Al3+ and its living cell imaging. Dalton Trans 44:4576–4581
Lee YJ, Lim C, Suh H, Song EJ, Kim C (2014) A multifunctional sensor: Chromogenic sensing for Mn2+ and fluorescent sensing for Zn2+ and Al3+. Sens Actuat B: Chem 201:535–544
Roy A, Nandi M, Roy P (2021) Dual chemosensors for metal ions: A comprehensive review. TrAC, Trends Anal Chem 138:116204
Ghorai A, Mondal J, Chandra R, Patra GK (2015) A reversible fluorescent-colorimetric imino-pyridyl bis-Schiff base sensor for expeditious detection of Al3+ and HSO3− in aqueous media. Dalton Trans 44:13261–13271
Chandra R, Kumar Manna A, Sahu M, Rout K, Patra GK (2020) Simple salicylaldimine-functionalized dipodal bis Schiff base chromogenic and fluorogenic chemosensors for selective and sensitive detection of Al3+ and Cr3+. Inorg Chim Acta 499:119192
Manna AK, Chowdhury S, Patra GK (2020) Combined experimental and theoretical studies on a phenyl thiadiazole-based novel turn-on fluorescent colorimetric Schiff base chemosensor for the selective and sensitive detection of Al3+. New J Chem 44:10819
Benramdane R, Benghanem F, Ourari A, Keraghel S, Bouet G (2015) Synthesis and characterization of a new Schiff base derived from 2,3-diaminopyridine and 5-methoxysalicylaldehyde and its Ni(II), Cu(II) and Zn(II) complexes. Electrochemical and electrocatalytical studies. J Coord Chem 68:560–572
Sarıgül M, Kariper SE, Deveci P, Atabey H, Karakaş D, Kurtoglu M (2017) Multi-properties of a new azo-Schiff base and its binuclear copper (II) chelate: Preparation, spectral characterization, electrochemical, potentiometric and modeling studies. J Mol Struct 1149:520–529
Kumar S, Hansda A, Chandra A, Kumar A, Kumar M, Sithambaresan M, Faizi SH, Kumar V, John RP (2017) Co(II), Ni(II), Cu(II) and Zn(II) complexes of acenaphthoquinone 3-(4-benzylpiperidyl)thiosemicarbazone: Synthesis, structural, electrochemical and antibacterial studies. Polyhedron 134:11–21
Funding
The authors thank Çanakkale Onsekiz Mart University scientific research project commission for support with the project number (Project Nu.: FBA-2021–3795).
Author information
Authors and Affiliations
Contributions
Diğdem Erdener: Writing-Reviewing and Editing, Investigation, Formal analysis, Writing-Original Draft, Methodology, Software. İsmet Kaya: Supervision, Resources, Conceptualization, Writing-Original Draft Methodology, Software.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erdener, D., Kaya, İ. Synthesis and Characterization of a Carbazole-Based Schiff Base Capable of Detection of Al3+ in Organic/Aqueous Media. J Fluoresc 32, 2097–2106 (2022). https://doi.org/10.1007/s10895-022-03008-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03008-y