Abstract
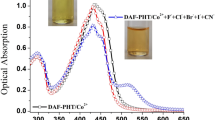
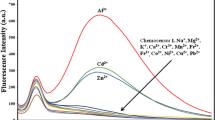
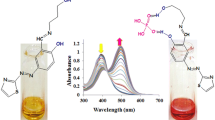
A series of new chemosensor molecules bearing benzothiazole-, quinoline- and phthalazine-functionalized fluorene derivatives were synthesized and their complexation behaviors with Fe3+ and Sn2+ ions were investigated. The sensing abilities of their complexes towards both cyanide and sulfide anions were investigated by colorimetric and fluorometric techniques in detail. The sensing mechanism was investigated by Job’s and Scatchard plots evaluations, and also absorption/fluorescence titration experiments. Among the studied dye/metal binary systems, F-BT sensor to Fe3+ giving the detection limits of 3.1 µg has also displayed high selectivity and sensitivity towards CN− and S2− anions, lead to a significant color change of the solution observable by the naked eye.













Similar content being viewed by others
Data Availability
Not applicable.
References
Liu W, Xu L, Sheng R, Wang P, Li H, Wu S (2007) A water-soluble “switching on” fluorescent Chemosensor of selectivity to Cd2+. Org Lett 9:3829–3832
Lee JH, Lim CS, Tian YS, Han JH, Cho BR (2010) A two-photon fluorescent probe for thiols in live cells and tissues. J Am Chem Soc 132:1216–1217
Zhang M, Yu M, Li F, Zhu M, Li M, Gao Y et al (2007) A highly selective fluorescence turn-on sensor for cysteine/homocysteine and its application in bioimaging. J Am Chem Soc 129:10322–10323
Amani V, Safari N, Khavasi HR, Mirzaei P (2007) Iron(III) mixed-ligand complexes: Synthesis, characterization and crystal structure determination of iron(III) hetero-ligand complexes containing 1,10-phenanthroline, 2,2′-bipyridine, chloride and dimethyl sulfoxide, [Fe(phen)Cl3(DMSO)] and [Fe(bipy)Cl3(DMSO)]. Polyhedron 26:4908–4914
Huang L, Hou F, Cheng J, Xi P, Chen F, Bai D et al (2012) Selective off-on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Org Biomol Chem 10:9634–9638
Lin W, Long L, Yuan L, Cao Z, Feng J (2009) A novel ratiometric fluorescent Fe3+ sensor based on a phenanthroimidazole chromophore. Anal Chim Acta 634:262–266
Wang H, Wang D, Wang Q, Li X, Schalley CA (2010) Nickel(II) and iron(III) selective off-on-type fluorescence probes based on perylene tetracarboxylic diimide. Org Biomol Chem 8:1017–1026
Wei P, Li D, Shi B, Wang Q, Huang F (2015) An anthracene-appended 2:3 copillar[5]arene: synthesis, computational studies, and application in highly selective fluorescence sensing for Fe(III) ions. Chem Commun 51:15169–15172
Gao Y, Liu H, Liu Q, Wang W (2016) A novel colorimetric and OFF–ON fluorescent chemosensor based on fluorescein derivative for the detection of Fe3+ in aqueous solution and living cells. Tetrahedron Lett 57:1852–1855
Tosonian S, Ruiz CJ, Rios A, Frias E, Eichler JF (2013) Synthesis, characterization, and stability of iron (III) complex ions possessing phenanthroline-based ligands. Open J Inorg Chem 3:7–13
Viau CM, Guecheva TN, Sousa FG, Pungartnik C, Brendel M, Saffi J et al (2009) SnCl2-induced DNA damage and repair inhibition of MMS-caused lesions in V79 Chinese hamster fibroblasts. Arch Toxicol 83:769–775
de Mattos JC, Lage C, Dantas FJ, Moraes MO, Nunes AP, Bezerra RJ et al (2005) Interaction of stannous chloride leads to alteration in DNA, triphosphate nucleotides and isolated bases. Mol Cell Biochem 280:173–179
El-Demerdash FM, Yousef MI, Zoheir MA (2005) Stannous chloride induces alterations in enzyme activities, lipid peroxidation and histopathology in male rabbit: antioxidant role of vitamin C. Food Chem Toxicol 43:1743–1752
Pungartnik C, Viau C, Picada J, Caldeira-de-Araujo A, Henriques JA, Brendel M (2005) Genotoxicity of stannous chloride in yeast and bacteria. Mutat Res 583:146–157
Yeates AT, Corredor CC, Belfield KD, Huang Z-L, Belfield KD, Kajzar F (2006) Two-photon FRET-based 3D optical data storage in a composite polymer containing a photochromic diarylethene and a fluorene dye. Proc of SPIE 6330:63300A
Belfield KD, Bondar MV, Przhonska OV, Schafer KJ (2004) Photochemical properties of (7-benzothiazol-2-yl-9,9-didecylfluoren-2-yl)diphenylamine under one- and two-photon excitation. J Photochem Photobiol A 162:569–574
Belfield KD, Bondar MV, Przhonska OV, Schafer KJ (2004) Photostability of a series of two-photon absorbing fluorene derivatives. J Photochem Photobiol A 162:489–496
Belfield KD, Bondar MV, Przhonska OV, Schafer KJ (2004) One- and two-photon photostability of 9,9-didecyl-2,7-bis(N,N-diphenylamino)fluorene. Photochem Photobiol S 3:138–141
Yao S, Ahn H-Y, Wang X, Fu J, Van Stryland EW, Hagan DJ et al (2010) Donor-acceptor-donor fluorene derivatives for two-photon fluorescence lysosomal imaging. J Org Chem 75:3965–3974
Erden İ, Erdoğmuş A, Demirhan N, Avcıata U (2008) Synthesis and characterization of a new fluorene ligand and its complexes with cobalt(II), copper(II), and ruthenium(II). Transit Metal Chem 33:439–442
Kumbar M, Patil SA, Toragalmath SS, Jawoor SS, Shettar A (2020) Synthesis of novel metal (II) complexes tailored from 9-oxo-9H-fluorene-1-carboxylic acid via green protocol: DNA cleavage and anticancer studies. Inorganica Chim Acta 500:119210
Zhu Q-Y, Lu W, Zhang Y, Bian G-Q, Gu J, Lin X-M et al (2008) Syntheses, crystal structures, and optical properties of metal complexes with 4′,5′-Diaza-9′-(4,5-disubstituted-1,3-dithiol-2-ylidene)fluorene ligands. Eur J Inorg Chem 2008:230–238
Belfield KD, Bondar MV, Frazer A, Morales AR, Kachkovsky OD, Mikhailov IA et al (2010) Fluorene-based metal-ion sensing probe with high sensitivity to Zn2+ and efficient two-photon absorption. J Phys Chem B 114:9313–9321
Kaushik R, Sakla R, Ghosh A, Dama S, Mittal A, Jose DA (2019) Copper complex-embedded vesicular receptor for selective detection of cyanide ion and colorimetric monitoring of enzymatic reaction. ACS Appl Mater Inter 11:47587–47595
Wang S-T, Chir J-L, Jhong Y, Wu A-T (2015) A turn-on fluorescent sensor for detection of cyanide in aqueous media. J Lumin 167:413–417
Wang K, Ma L, Liu G, Cao D, Guan R, Liu Z (2016) Two fluorescence turn-on coumarin Schiff’s base chemosensors for cyanide anions. Dyes Pigments 126:104–109
Wang L, Li L, Cao D (2016) Dual binding site assisted chromogenic and fluorogenic discrimination of fluoride and cyanide by boryl functionalized BODIPY. Sensor Actuat B Chem 228:347–359
Gore AH, Vatre SB, Anbhule PV, Han SH, Patil SR, Kolekar GB (2013) Direct detection of sulfide ions S2- in aqueous media based on fluorescence quenching of functionalized CdS QDs at trace levels: analytical applications to environmental analysis. Analyst 138:1329–1333
Ryu S-J, Arifin E, Ha S-W, Lee J-K (2015) On-site colorimetric forensic sensor (II): quantitative detection of toxic S2- ion in blood plasma using metal-ion-modified silica powders. Bull Korean Chem Soc 36:2506–2510
Paul A, Anbu S, Sharma G, Kuznetsov ML, Guedes da Silva MF, Koch B et al (2015) Intracellular detection of Cu2+ and S2- ions through a quinazoline functionalized benzimidazole-based new fluorogenic differential chemosensor. Dalton Trans 44:16953–16964
Pommerehne J, Vestweber H, Guss W, Mahrt RF, Bassler H, Porsch M et al (1995) Efficient 2-layer leds on a polymer blend basis. Adv Mater 7:551–554
Facchiano A, Ragone R (2003) Modification of Job’s method for determining the stoichiometry of protein–protein complexes. Anal Biochem 313:170–172
Renny JS, Tomasevich LL, Tallmadge EH, Collum DB (2013) Method of continuous variations: applications of job plots to the study of molecular associations in organometallic chemistry. Angew Chem 52:11998–12013
Dias FB, Pollock S, Hedley G, Pålsson L-O, Monkman A, Perepichka II et al (2006) Intramolecular charge transfer assisted by conformational changes in the excited state of fluorene-dibenzothiophene-S,S-dioxide co-oligomers. J Phys Chem B 110:19329–19339
Mysyk DD, Perepichka IF (1994) Fluorene compounds with intramolecular charge transfer containing dithiolylidene and selenathiolylidene substituents. Phosphorus Sulfur 95:527–529
Perepichka IF, Perepichka D, Popov A, Perepichka I, Bryce RM, Goldenberg ML, et al (1998) Fluorene acceptors with intramolecular charge-transfer from 1,3-dithiole donor moieties: novel electron transport materials. Chem Commun 7:819–820
Perepichka DF, Perepichka IF, Ivasenko O, Moore AJ, Bryce MR, Kuz’mina LG et al (2008) Combining high electron affinity and intramolecular charge transfer in 1,3-Dithiole–Nitrofluorene push–pull diads. Chem Eur J 14:2757–2770
Shigeta M, Morita M, Konishi G (2012) Selective formation of twisted intramolecular charge transfer and excimer emissions on 2,7-bis(4-diethylaminophenyl)-fluorenone by choice of solvent. Molecules 17:4452–4459
Song S, Ju D, Li J, Li D, Wei Y, Dong C et al (2009) Synthesis and spectral characteristics of two novel intramolecular charge transfer fluorescent dyes. Talanta 77:1707–1714
Murphy RS, Moorlag CP, Green WH, Bohne C (1997) Photophysical characterization of fluorenone derivatives. J Photochem Photobiol A 110:123–129
Jung HJ, Singh N, Jang DO (2008) Highly Fe3+ selective ratiometric fluorescent probe based on imine-linked benzimidazole. Tetrahedron Lett 49:2960–2964
Singh N, Lee GW, Jang DO (2008) p-tert-Butylcalix[4]arene-based fluororeceptor for the recognition of dicarboxylates. Tetrahedron 64:1482–1486
Singh N, Lee GW, Jang DO (2008) Highly selective imine-linked fluorescent chemosensor for adenine employing multiple hydrogen bonding. Tetrahedron Lett 49:44–47
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2009) Gaussian 09. Gaussian, Inc., Wallingford
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Acknowledgements
This work was supported by the Research Council of Manisa Celal Bayar University with the project number of 2018 − 126.
Funding
The funder has been acknowledged in the acknowledgement section.
Author information
Authors and Affiliations
Contributions
Gözde Murat Saltan and Haluk Dinçalp made substantial contribution while preparing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Not applicable.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 2
Cyclic voltammograms of F-PHT, F-BT, and F-QU dyes on glassy carbon working electrode in 0.1 M [TBA][PF6]/MeCN (Scan rate: 100 mV s─1) (JPG 523 kb)
ESM 3
Optical absorption changes of (a) F-PHT (2 × 10−5 M) and (b) F-QU (2 × 10−5 M in MeCN solution in the presence of different metal ions at saturated concentrations (JPG 717 kb)
ESM 4
(JPG 742 kb)
ESM 5
Changes in UV–vis absorption and fluorescence emission spectra (inset) of F-PHT at the initial concentrations of 2 × 10−5 M in MeCN solution by the titration upon addition of (a) Sn2+ (0 to 26.0 × 10−5 M), and (b) Fe3+ ions (0 to 42.0 × 10−6 M) indicated by arrow (λexc = 375 nm) (JPG 682 kb)
ESM 6
(JPG 738 kb)
ESM 7
Changes in UV–vis absorption and fluorescence emission spectra (inset) of F-QU at the initial concentrations of 2 × 10−5 M in MeCN solution by the titration upon addition of (a) Sn2+ (0 to 26.0 × 10−5 M), and (b) Fe3+ ions (0 to 42.0 × 10−6 M) indicated by arrow (λexc = 375 nm) (JPG 698 kb)
ESM 8
(JPG 682 kb)
ESM 9
Job’s plots for (a) F-PHT/Sn2+, (b) F-PHT/Fe3+, (c) F-QU/Sn2+, (d) F-BT/Sn2+, (e) F-BT/Fe3+ complex in MeCN solution (The total concentration of each was 2 × 10−5 M) (JPG 450 kb)
ESM 10
(JPG 431 kb)
ESM 11
(JPG 446 kb)
ESM 12
(JPG 471 kb)
ESM 13
(JPG 422 kb)
ESM 14
Scatchard plot for (a) F-PHT/Sn2+ (F−F0/[Sn2+] = −7.33 × 103(F − F0) + 6.5 × 107; R2: 0.89), and (b) F-BT/Sn2+ (F−F0/[Sn2+] = −2.11 × 103(F − F0) + 2.4 × 107; R2: 0.94) complex in MeCN solution (JPG 426 kb)
ESM 15
(JPG 406 kb)
Rights and permissions
About this article
Cite this article
Murat Saltan, G., Dinçalp, H. Fluorene Based Ferric Complex as Colorimetric and Fluorometric Probe for Highly Selective Detection of CN− and S2− Anions. J Fluoresc 31, 1311–1321 (2021). https://doi.org/10.1007/s10895-021-02737-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02737-w