Abstract
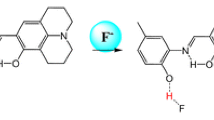
A simple colorimetric and turn-on fluorescence receptor FT (thiophene appended fluorescein-hydrazone derivative) was prepared and its cation-sensing properties were investigated. Receptor FT displayed a selective colorimetric change (from colorless to orange color) upon binding to Hg2+ in DMSO/H2O (1:9, v/v) solution. The association constant of FT-Hg2+ complex was calculated to be 3.03 × 109 M−1, and the detection limit for Hg2+ was found to be 0.24 ppm.










Similar content being viewed by others
References
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Chem Rev 97:1515–1566
McKeown-Eyssen GE, Ruedy J, Neims A (1983) Am J Epidemol 118:470–479
Davidson PW, Myers GJ, Cox C, Shamlaye CF, Marsh DO, Tanner MA, Berlin M, Sloane-Reeves J, Cernichiari E, Choisy O, Choi A, Clarkson TW (1995) Neurotoxicology 16:677–688
Grandjean P, Weihe P, White RF, Debes F (1998) Environ Res 77:165–172
Yuan M, Li Y, Li J, Li C, Liu X, Lv J, Xu J, Liu H, Wang S, Zhu D (2007) Org Lett 9:2313–2316
Zhu M, Yuan M, Liu X, Xu J, Lv J, Huang C, Liu H, Li Y, Wang S, Zhu D (2008) Org Lett 10:1481–1484
Kao T-L, Wang C-C, Pan Y-T, Shiao Y-J, Yen J-Y, Shu C-M, Lee G-H, Peng S-M, Chung W-S (2005) J Org Chem 70:2912–2920
Chen Q-Y, Chen CF (2005) Tetrahedron Lett 46:165–168
Moon SY, Cha NR, Kim YH, Chang SK J. Org. Chem 69(2004):181–183
Cha NR, Kim MY, Kim YH, Choe J-I, Chang S-K, Chem J (2002) Soc Perkin Trans 2:1193–1196
Kumar A, Pandey PS (2009) Tetrahedron Lett 50:5842–5845
Chen X, Nam SW, Jou MJ, Kim Y, Kim S, Park S, Yoon J (2008) Org Lett 10:5235–5238
Zhang X, Xiao Y, Qian X (2008) Angew Chem Int Ed 47:8025–8029
Xiang Y, Tong A, Jin P, Ju Y (2006) Org Lett 8:2863–2866
Zhang X, Shiraishi Y, Hirai T (2007) Org Lett 9:5039–5042
Lee MH, Kim HJ, Yoon S, Park N, Kim JS (2008) Org Lett 10:213–216
Yang XF, Liu P, Wang L, Zhao M (2008) J Fluoresc 18:453–459
Wu JS, Hwang IC, Kim KS, Kim Org JS (2007) Lett 9:907–910
Yang YK, Yook KJ, Tae J (2005) J Am Chem Soc 127:16760–16761
Ko SK, Yang YK, Tae J, Shin I (2006) J Am Chem Soc 128:14150–14155
Yang H, Zhou Z, Huang K, Yu M, Li F, Yi T (2007) Org Lett 9:4729–4732
Soh JH, Swamy KMK, Kim SK, Kim S, Lee SH, Yoon J (2007) Tetrahedron Lett 48:5966–5969
Yang YK, Yook KJ, Tae J J. Am. Chem. Soc 127(2005):16760–16761
Lin W, Cao X, Ding Y, Yuan L, Long L (2010) Chem Commun 46:3529–3531
Adhikari S, Ghosh A, Mandal S, Sengupta A, Chattopadhyay A, Matalobos JS, Lohar S, Das D (2014) Dalton Trans 43:7747–7751
Kroll H (1952) J Am Chem Soc 74:2036–2039
Bender ML, Turnquist BW (1957) J Am Chem Soc 79:1889–1893
Mao Y, Hong M, Liu A, X D (2015) J Fluoresc 25:755–761
Anand T, Sivaraman G, Chellappa D (2014) J Photoch Photobiol A 281:47–52
Dujols V, Ford F, Czarnik AW (1997) J Am Chem Soc 119:7386–7387
Chen XQ, Ma HM (2006) Anal Chem Acta 575:217–222
Tian MZ, Hu MM, Fan JL, Peng XJ, Wang JY, Sun SG, Zhang R (2013) Bioorg Med Chem Lett 23:2916–2919
Acknowledgments
Ministry of Science and Technology, R. O. C. (grant numbers: 104-2113-M-018-002).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
The experimental procedures and the 1H NMR, 13C NMR and ESI-MS spectra are available. Supplementary data associated with this article can be found.
Fig. S1
(DOC 96 kb)
Fig. S2
(DOC 136 kb)
Fig. S3
(DOC 110 kb)
Fig. S4
(DOC 96 kb)
Fig. S5
(DOC 59 kb)
Fig. S6
(DOC 59 kb)
Fig. S7
(DOC 45 kb)
Fig. S8
(DOC 69 kb)
Fig. S9
(DOC 48 kb)
Fig. S10
(DOC 121 kb)
Fig. S11
(DOC 274 kb)
Fig. S12
(DOC 85 kb)
Rights and permissions
About this article
Cite this article
Chiou, YR., Wan, CF. & Wu, AT. A Selective Colorimetric and Turn-on Fluorescent Chemosensor for Hg2+ in Aqueous Solution. J Fluoresc 27, 317–322 (2017). https://doi.org/10.1007/s10895-016-1960-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1960-7