Abstract
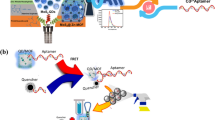
An aqueous suspension of fluorescent nanoparticles (PHNNPs) of naphthol based fluorescent organic compound 1-[(Z)-(2-phenylhydrazinylidene) methyl] naphthalene −2-ol (PHN) were prepared using reprecipitation method shows bathochromically shifted aggregation induced enhanced emission (AIEE) in the spectral region where erythrosine (ETS) food dye absorbs strongly. The average size of 72.6 nm of aqueous suspension of PHNNPs obtained by Dynamic light scattering results shows a narrow particle size distribution. The negative zeta potential of nano probe (−22.6 mV) responsible to adsorb oppositely charged analyte on its surface and further permit to bind nano probe and analyte within the close distance proximity required for efficient fluorescence resonance energy transfer (FRET) to take place from donor (PHNNPs) to acceptor (ETS). Systematic FRET experiments performed by measuring fluorescence quenching of PHNNPs with successive addition of ETS solution exploited the use of the PHNNPs as a novel nano probe for the detection of ETS in aqueous solution with extremely lower limit of detection equal to 3.6 nM (3.1 ng/mL). The estimation of photo kinetic and thermodynamic parameters such as quenching rate constant, enthalpy change (∆H), Gibbs free energy change (∆G) and entropy change (∆S) was obtained by the quenching results obtained at different constant temperatures which were found to fit the well-known Stern–Volmer relation. The mechanism of binding and fluorescence quenching of PHNNPs by ETS food dye is proposed on the basis of results obtained in photophysical studies, thermodynamic parameter, energy transfer efficiency, critical energy transfer distance (R0) and distance of approach between donor–acceptor molecules (r). The proposed FRET method based on fluorescence quenching of PHNNPs was successfully applied to develop an analytical method for estimation of ETS from food stuffs without interference of other complex ingredients.

A fluorescent organic nanoprobe developed for the detection of erythrosine (ETS) food dye in aqueous medium based on fluorescence resonance energy transfer (FRET). The FRET process between donor (nanoparticles) and acceptor (ETS dye) arises due to oppositely charge attraction through hydrophobic interactions. The proposed method was successfully applied to quantitative determination of ETS dye in food stuff sample collected from local market.















Similar content being viewed by others
References
Peska V, Jahn M, Stolcova L, Schulz V, Proska J, Prochazka M, Weber K, Cialla-May D, Popp J (2015) Quantitative SERS analysis of Azorubine (E 122) in sweet drinks. Anal Chem 87:2840–2844
Vinas P, Pardo-Martianez M, Pez-Garciaa I, Hernandezcordoba M (2002) Rapid determination of mercury in food colorants using Electrothermal atomic absorption spectrometry with slurry sample introduction. J Agric Food Chem 50:949–954
Sharma V, McKone H, Markow P (2011) A global perspective on the history, use, and identification of synthetic food dyes. J Chem Educ 88:24–28
Guo J, Wu H, Du L, Fu Y (2013) Determination of brilliant blue FCF in food and cosmetic samples by ionic liquid independent disperse liquid–liquid micro-extraction. Anal Methods 5:4021–4026
Ramakrishnan SP, Bagya Lakshmi L, Surya PR (2011) Estimation of synthetic dye erythrosine in food stuff and formulation and effect of dye on the protein binding of drug in BSA. Der Pharmacia Lettre 3:361–373
Kaur A, Gupta U (2014) Preconcentration of erythrosine dye using β-cyclodextrin epichlorohydrin polymer as a solid phase extractant. World J Pharm Pharm Sci 4:812–819
Bhopate DP, Mahajan PG, Garadkar KM, Kolekar GB, Patil SR (2015) Polyvinyl pyrrolidone capped fluorescent anthracene nanoparticles for sensing fluorescein sodium in aqueous solution and analytical application for ophthalmic samples. Luminesce. doi:10.1002/bio.2858
Ordoudi SA, Tsimidou MZ (2011) Consideration of fluorescence properties for the direct determination of erythrosine in saffron in the presence of other synthetic dyes. Food Addit Contam 28:417–422
Yuan L, Lin W, Zheng K, Zhu S (2013) FRET-based small-molecule fluorescent probes: rational design and Bioimaging applications. Acc Chem Res 6:1462–1473
Zhang H, Liu R, Tan Y, Haowei Xie W, Lei H, Cheung H, Sun H (2015) A FRET - based ratiometric fluorescent probe for Nitroxyl detection in living cells. ACS Appl Mater Interfaces 7:5438–5443
Bhattar SL, Kolekar GB, Patil SR (2011) FRET between anthracene and Proflavine Hemisulphate in micellar solution and analytical application on determination of Proflavine Hemisulphate. J Dispers Sci Technol 32:23–27
Bhattar SL, Kolekar GB, Patil SR (2008) Fluorescence resonance energy transfer between perylene and riboflavin in micellar solution and analytical application on determination of vitamin B2. J Lumin 128:306–310
Saha J, Datta Roy A, Dey D, Chakraborty S, Bhattacharjee D, Paul PK, Hussain SA (2015) Investigation of fluorescence resonance energy transfer between fluorescein and rhodamine 6G. Spectrochim Acta A Mol Biomol Spectrosc 149:143–149
Dalavi DK, Kamble AA, Bhopate DP, Mahajan PG, Kolekar GB, Patil SR (2015) TNPs as a novel fluorescent sensor for the selective recognition of fast green FCF: a spectrofluorimetric approach. RSC Adv 5:69371–69377
Dige NC, Pore DM (2015) Green aspect for multicomponent synthesis of spiro[4H-indeno[1,2-b]pyridine-4,3′-[3H]indoles]. Syn comm 45: 2498–2510
Tunç T, Tezcan H, Saglam S, Dilek N (2014) Comparative experimental and theoretical studies ofN-(4-Methylbenzylidene)-N0-(2-carboxyphenyl) hydrazine novel Schiff base. Spectrochim Acta Part A: Mol Biomol Spectro 127:490–497
Das DK, Goswami P, Sarma P (2013) A new highly selective fluorescence lead (II) probe. J Fluoresc 23:503–508
Ghosh S, Alam A, Gangulya A, Guchhait N (2015) A new highly selective fluorescence lead (II) probe. Spectrochim Acta Part A: Mol Biomol Spectro 149:869–874
Mahajan PG, Desai NK, Dalavi DK, Bhopate DP, Kolekar GB, Patil SR (2015) Cetyltrimethylammonium bromide capped 9-Anthraldehyde nanoparticles for selective recognition of phosphate anion in aqueous solution based on fluorescence quenching and application for analysis of chloroquine. J Fluoresc 25:31–38
Wang F, Han M, Yi Mya K, Wang Y, Lai Y (2005) Aggregation driven growth of size-tunable organic nanoparticles using electronically altered conjugated polymers. J Am Chem Soc 127:10350–10355
Foster T, Sinanoglu O (1996) Quantum chemistry, vol 3. Academic Press, New York, p. 93
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Plenum Press, New York
Mahajan PG, Bhopate DP, Kamble AA, Dalavi DK, Kolekar GB, Patil SR (2015) Selective sensing of Fe2+ ions in aqueous solution based on fluorescence quenching of SDS capped rubrene nanoparticles: application in pharmaceutical formulation. Anal Methods 7:7889–7898
Forster T (1959) 10th Spiers memorial lecture. Transfer mechanisms of electronic excitation. Discuss Faraday Soc 27:7–17
Chen G, Song F, Xiong X, Peng P (2013) Fluorescent nanosensors based on fluorescence resonance energy transfer (FRET). Ind Eng Chem Res 52:11228–11245
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20:3096–3102
Hu Y, Liu Y, Zhao RM, Dong JX, Qu SS (2006) Spectroscopic studies on the interaction between methylene blue and bovine serum albumin. Photochem Photobiol A 179:324–329
Jiang XY, Li WX, Cao H (2008) Study of the interaction between trans-resveratrol and BSA by the multi-spectroscopic method. J Solut Chem 37:1609–1623
Wangoo N, Kaushal J, Bhasin KK, Mehta SK, Suri CR (2010) Zeta potential based colorimetric immunoassay for the direct detection of diabetic marker HbA1c using gold nanoprobes. Chem Commun 46:5755–5757
Teng Y, Jia X, Li J, Wang E (2015) Ratiometric fluorescence detection of Tyrosinase activity and dopamine using thiolate-protected gold nanoclusters. Anal Chem 87:4897–4902
Zhang K, Yu T, Liu F, Sun M, Yu H, Liu B, Zhang Z, Jiang H, Wang S (2014) Selective fluorescence turn-on and ratiometric detection of organophosphate using dual-emitting Mn-doped ZnS nanocrystal probe. Anal Chem 86:11727–11733
Albers AE, Okreglak VS, Chang CJ (2006) A FRET-based approach to ratiometric fluorescence detection of hydrogen peroxide. J Am Chem Soc 128:9640–9641
Mahajan PG, Bhopate DP, Kolekar GB, Patil SR (2015) N-methyl isatin nanoparticles as a novel probe for selective detection of Cd2+ ion in aqueous medium based on chelation enhanced fluorescence and application to environmental sample. Sensors Actuators B 220:864–872
Trandafir I, Nour V, Ionica M (2009) The liquid chromatographic quantification of some synthetic colorants in soft drinks. Sci Stu Res 1:73–82
Minioti KS, Sakellariou CF, Thomaidis NS (2007) Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal Chim Acta 583:103–110
Kaur A, Gupta U (2012) Simultaneous spectrophotometric Determiantion of eosin and erythrosine with Cr (VI) reagent in micellar media using mean centering of ratio spectra. Chem Sci Trans 1:424–430
Kaur A, Gupta U (2012) Simultaneous spectrophotometric determination of eosin and erythrosine in pharmaceutical and food samples by using mean centering of ratio spectra method. Int J Res Chem Environ 2:55–62
Acknowledgments
One of the authors PGM is grateful to the University Grants Commission (UGC), New Delhi for providing financial assistance in the form of UGC-BSR-SAP fellowship (F.25-1/2013-14(BSR)/7-183/2007(BSR)- May 2014). We are also grateful to the Department of Science and Technology (DST), New Delhi for providing funds under FIST-Level-II program for infrastructure improvement and University Grants Commission (UGC), New Delhi for financial support through DRS - Phase- II program to the Department of Chemistry, Shivaji University, Kolhapur.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 901 kb)
Rights and permissions
About this article
Cite this article
Mahajan, P.G., Bhopate, D.P., Kolekar, G.B. et al. FRET Sensor for Erythrosine Dye Based on Organic Nanoparticles: Application to Analysis of Food Stuff. J Fluoresc 26, 1467–1478 (2016). https://doi.org/10.1007/s10895-016-1839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1839-7