Abstract
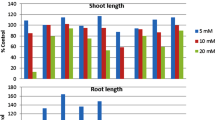
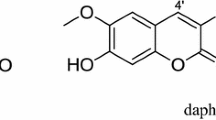
Nine natural plant compounds were screened for phytotoxicity to Bidens pilosa L. a troublesome weed in field and plantation crops. The sensitivity of three other weed species to coumarin, the most active identified compound, was also evaluated. Coumarin, at a concentration of 500 μM, had little effect on germination and growth of Senna obtusifolia L., Euphorbia heterophylla L., and Ipomoea grandifolia L. when compared with its effects on B. pilosa L. In a concentration range of 10–100 μM, coumarin caused a dose-dependent inhibition of germination and growth of B. pilosa L. The measurements of some parameters of energy metabolism revealed that coumarin-treated root tissues exhibited characteristics of seedlings in an earlier stage of growth, including higher respiratory activity and higher activities of alcohol dehydrogenase and lipoxygenase. These results suggest that coumarin inhibition of germination and growth of B. pilosa L. was not a consequence of an impairment of energy metabolism. Rather, it seems to act as a cytostatic agent, retarding germination. At concentrations above 50 μM, coumarin increased lipoxygenase activity and the level of conjugated dienes of root extracts, suggesting that it may induce oxidative stress in seedling roots.



Similar content being viewed by others
Abbreviations
- AOX:
-
alternative oxidase
- COX:
-
cytochrome oxidase
- DTT:
-
dithiothreitol
- EDTA:
-
ethylene diamide tetracetic acid
- KCN:
-
potassium cyanide
- MDA:
-
malondialdehyde
- TBA:
-
2-thiobarbituric acid
- TCA:
-
trichloroacetic acid
References
Abenavoli, M. R., Sorgonã, A., Sidari, M., Badiani, M., and Fuggi, A 2003. Coumarin inhibits the growth of carrot (Daucus carota L. cv. Saint Valery) cells in suspension culture. J. Plant Physiol. 160:227–237.
Abenavoli, M. R., Sorgonà, A., Sidari, M., Albano, A., and Cacco, G. 2004. Coumarin differentially affects the morphology of different root types of maize seedlings. J. Chem. Ecol. 30:1871–1883.
Abenavoli, M. R., Cacco, G., Sorgonà, A., Marabottini, R., Paolacci, A. R., Ciaffi, M., and Badiani, M. 2006. The inhibitory effect of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, CV. SIMETO) seeds. J. Chem. Ecol. 32:489–506.
Beuge, J. A., and Aust, S. D. 1978. Microsomal lipid peroxidation. Method. Enzymol. 52:302–310.
Blokhina, O., Virolainen, E., and Fagersted, V. K. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91:179–194.
Boveris, A., Cadenas, E., and Chance, B. 1980. Low level chemiluminescence of the lipoxygenase reaction. Photobiochem. Photobiophys. 1:175–182.
Chon, S. U., and Kim, Y. M. 2004. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agron. Crop Sci. 190:145–150.
Chon, S. U., Kim, Y. M., and Lee, J. C. 2003. Herbicidal potencial and quantification of causative allelochemicals from several Compositae weeds. Weed Res. 43:444–450.
Christoffoleti, P. J., and Foloni, L. 1999. Dose-response curves of resistant and susceptible Bidens pilosa to ALS inhibitor herbicide, pp. 159–162, in Proceedings from the Brighton Crop Protection Conference: weeds. November, 1999, London, UK.
Dudai, N., Poljakoff-Mayber, A., Mayer, A. M., Putievsky, E., and Lerner, H. R. 1999. Essential oils as allelochemical and their potential use as bioherbicides. J. Chem. Ecol. 25:079–1089.
Duke, S. O., Dayan, F. E., omagni, J. G., and imando, A. M. 2000. Natural products as sources of herbicides: current status and future trends. Weed Res. 40:99–111.
Estabrook, R. W. 1967. Mitochondrial respiratory control and polarographic measurements of ADP/O ratio. Method. Enzymol. 10:41–47.
Fischer, N. H., Williamson, G. B., Weidenhamer, J. D., and Richardson 1994. In search of allelopathy in the Florida scrub: the role of terpenoids. J. Chem. Ecol. 20:1355–1379.
Hara, M., Umetsu, N., Miyamoto, C., and Tamari, K. 1973. Inhibition of the biosynthesis of plant cell wall materials, especially cellulose biosynthesis by coumarin. Plant Cell Physiol. 14:11–28.
Heath, R. L., and Packer, L. 1968. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acids peroxidation. Arch. Biochem. Biophys. 125:189–198.
Holm, L. G., Plucknett, D. L., Pancho, J. V., and Herberger, J. P. 1977. The World’s Worst Weeds: Distribution and Biology. The University Press of Hawaii, Honolulu.
Jansson, E., and Svensson, S. B. 1980. Coumarin effects on Glycine max hypocotyls explants. Physiol. Plantarum 48:486–490.
Khanh, T. D., Chung, I. M., Tawata, S., and Xuan, T. D. 2006. Weed suppression by Passiflora edulis and its potential allelochemicals. Weed Res. 46:296–303.
Knypl, J. S. 1964. Coumarin-induced respiration of sunflower. Physiol. Plantarum 17:771–778.
Kohli, R. K., Batish, D. R., and Singh, H. P. 2006. Allelopathic interactions in agroecosystems, pp. 465–493, in M. V. Reigosa, N. Pedrol, and L. González (eds.). Allelopathy: A Physiological Process with Ecological Implications Springer, Netherlands.
Kupidlowska, E., Kowalec, M., Sulkowski, G., and Zobel, A. M. 1994. The effects of coumarin on root elongation and ultrastructure of meristematic cell protoplast. Ann. Bot. 73:525–530.
Labouriau, L. G., and Osborn, J. H. 1984. Temperature dependence of the germination of tomato seeds. J. Therm. Biol. 9:285–294.
Larkin, P. J. 1987. Calmodulin levels are not responsible for aluminum tolerance in wheat. Aust. J. Plant Physiol. 14:377–387.
Lee, C. Y. 1982. Alcohol dehydrogenase from Drosophila melanogaster. Method. Enzymol. 89:445–450.
Macías, F. 1995. Allelopathy in the search for natural herbicide models, pp. 310–329, in Inderjit, M. M. Dakshini, and F. A. Einhellig (eds.). Allelopathy: Organisms, Processes, and Applications American Chemical Society, Washington DC.
Macías, F., Galindo, J. C. G., Massanet, G. M., Rodriguez-Luís, F., and Zubías, E. 1993. Allelochemicals from Pilocarpus goudotianus leaves. J. Chem. Ecol. 19:1371–1379.
Moreland, E. D., and Novitzky, W. P. 1987. Effects of coumarins and flavonoids on isolated chloroplast and mitochondria, pp. 247–261, in G. R. Waller (ed.). Allelochemicals: Role in Agriculture and ForestryAmerican Society Series. Americal Chemical Society, Washington, DC.
Murray, R. D. H., Méndez, J., and Brown, S. A. 1982. The Natural Coumarins: Occurrence, Chemistry and Biochemistry. J. Wiley and Sons, Chichester.
Muscari, C. L., Frascaro, M., Guarnieri, C., and Caldarera, C. I. M. 1990. Mitochondrial function and superoxide generation from submitochondrial particles of aged rat hearts. Biochim. Biophys. Acta 1015:200–204.
Podbiekowska, M., Kupidlowska, E., Waleza, M., Dobrzynska, K., Louis, S. A., Keightley, A., and Zobel, A. M. 1994. Coumarin as antimitotics. Int. J. Pharm. 32:262–273.
Porta, H., and Rocha-Sosa, M. 2002. Plant lipoxygenases. Physiological and Molecular Features. Plant Physiol. 130:15–21.
Putnam, A. R., and Duke, W. B. 1974. Biological suppression of weeds: evidence for allelopathy in accessions of cucumber. Science 185:370–372.
Reigosa, M. J., and Pazos-Malvido, E. 2007. Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem Ecol. 33:1456–1466.
Reigosa, M. J., Souto, X. C., and González, L. 1999. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Reg. 28:83–88.
Richard, H., Goodwin, A. E., and Taves, C. 1950. The effect of coumarin derivatives on the growth of Avena roots. Amer. J. Bot. 37:224–231.
Siedow, J. N. 1991. Plant lipoxygenase: structure and function. Annu. Rev. Plant Physiol. 42:145–188.
Siedow, J. N., and Girvin, M. E. 1980. Alternative respiratory pathway. Plant Physiol. 65:669–674.
Svensson, S. B. 1972. The effect of coumarin on growth, production of dry matter, protein and nucleic acids in roots of maize and wheat and the interactions of coumarin with metabolic inhibitors. Physiol. Plantarum 27:13–24.
Van Sumere, C. F., Cottenie, J., De Greef, J., and Kint, J. 1972. Biochemical studies in relation to the possible germination regulatory role of naturally occurring coumarin and phenolic. Recent Adv. Phytochemistry 4:165–221.
Vaughn, S. F., and Spencer, G. F. 1993. Volatile monoterpenes as potential parent structures for new herbicides. Weed Sci. 41:114–119.
Vyvyan, J. R. 2002. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 58:1631–1646.
Yakushkina, N. I., and Starikova, V. T. 1978. Effects of coumarin and gibberellin on certain aspects of the energy metabolism of corn seedlings. Fiziol. Rast. 24:1211–1216.
Wu, H., Pratley, J., Lemerle, D., and Haig, T. 1999. Crop cultivars with allelopathic capability. Weed Res. 39:171–180.
Zobel, A. M., and Brown, S. A. 1995. Coumarins in the interactions between the plant and its environment. Allelopathy J. 2:9–20.
Acknowledgements
This work was supported by grants from the Fundação Araucária do Estado do Paraná and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Érica Marusa Pergo fellowship holder from the Conselho Nacional de Desenvolvimento Científico e Tecnológico. We are indebted to Dr. Adelar Bracht for suggestions on the revision of the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pergo, É.M., Abrahim, D., Soares da Silva, P.C. et al. Bidens pilosa L. Exhibits High Sensitivity to Coumarin in Comparison with Three Other Weed Species. J Chem Ecol 34, 499–507 (2008). https://doi.org/10.1007/s10886-008-9449-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9449-8