Abstract
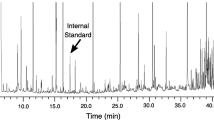
A growing body of evidence indicates that odors are used in individual, sexual, and species recognition in vertebrates, and may be reliable signals of quality and compatibility. Petrels are seabirds that exhibit an acute sense of smell. During the breeding period, many species of petrel live in dense colonies on small oceanic islands and form pairs that use individual underground burrows. Mates alternate between parental duties and foraging trips at sea. Returning from the ocean at night (to avoid bird predators), petrels must find their nest burrow. Antarctic prions, Pachyptila desolata, are thought to identify their nest by recognizing their partner’s odor, suggesting the existence of an individual odor signature. We used gas chromatography and mass spectrometry to analyze extracts obtained from the feathers of 13 birds. The chemical profile of a single bird was more similar to itself, from year to year, than to that of any other bird. The profile contained up to a hundred volatile lipids, but the odor signature may be based on the presence or absence of a few specific compounds. Our results show that the odor signature in Antarctic prions is probably endogenous, suggesting that in some species of petrels it may broadcast compatibility and quality of potential mates.


Similar content being viewed by others
References
Adams, R. P. 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publishing Corporation, Carol Stream, IL.
Alberts, A. C. 1992. Constraints on the design of chemical communication-systems in terrestrial vertebrates. Am. Nat. 139:S62–S89.
Balthazart, J., and Schoffeniels, E. 1979. Pheromones are involved in the control of sexual behavior in birds. Naturwissenschaften 66:55–56.
Bang, B. G. 1966. The olfactory apparatus of tubenosed birds (Procellariiformes). Acta Anat. 65:391–415.
Bang, B. G., and Wenzel, B. M. 1985. Nasal cavity and olfactory system, pp. 195–225, in A. S. King and J. McLelland (eds.). Form and Function in Birds, vol. 5. Academic, London.
Bonadonna, F., and Nevitt, G. A. 2004. Partner-specific odor recognition in an Antarctic seabird. Science 306:835.
Bonadonna, F., Hesters, F., and Jouventin, P. 2003. Scent of a nest: discrimination of own-nest odours in Antarctic prions, Pachyptila desolata. Behav. Ecol. Sociobiol. 54:174–178.
Bonadonna, F., Villafane, M., Bajzak, C., and Jouventin, P. 2004. Recognition of burrow’s “olfactory signature” in blue petrels, Halobaena caerulea: an efficient discrimination mechanism in the dark. Anim. Behav. 67:893–898.
Burger, B. V. 2005. Mammalian semiochemicals. Chemistry of Pheromones and Other Semiochemicals II 240:231–278.
Burger, B. V., Tien, F. C., Le Roux, M., and Mo, W. P. 1996. Mammalian exocrine secretions.10. Constituents of preorbital secretion of grysbok, Raphicencs melanotis. J. Chem. Ecol. 22:739–764.
Burger, B. V., Nell, A. E, Spies, H. S. C., Le Roux, M., Bigalke, R. C., and Brand, P. A. J. 1999. Mammalian exocrine secretions. XII: constituents of interdigital secretions of bontebok, Damaliscus dorcas dorcas, and blesbok, D-d. phillipsi. J. Chem. Ecol. 25:2057–2084.
Burger, B. V., Reiter, B., Borzyk, O., and Du Plessis, M. A. 2004. Avian exocrine secretions. I. Chemical characterization of the volatile fraction of the uropygial secretion of the green woodhoopoe, Phoeniculus purpureus. J. Chem. Ecol. 30:1603–1611.
Gassett, J. W., Wiesler, D. P., Baker, A. G., Osborn, D. A., Miller, K. V., Marchinton, R. L., and Novotny, M. 1997. Volatile compounds from the forehead region of male white-tailed deer (Odocoileus virginianus). J. Chem. Ecol. 23:569–578.
Grubb, T. C. 1972. Smell and foraging in shearwaters and petrels. Nature 237:404–405.
Hagelin, J. C., Jones, I. J., and Rasmussen, L. E. L. 2003. A tangerine-scented social odour in a monogamous seabird. Proc. R. Soc. Lond. B 270:1323–1329.
Jacob, J., and Hoerschelmann, H. 1982. Chemotaxonomische Untersuchungen Zur Systematik Der Rohrennasen (Procellariiformes). J. Ornithol. 123:63–84.
Jacob, J., and Ziswiler, V. 1982. The Uropygial Gland, pp. 199–324, in D. S. Farner, J. R. King, K. C. Parkes (eds). Avian Biology, vol. 6. Academic, New York.
Jacob, J., Balthazart, J., and Schoffeniels, E. 1979. Sex differences in the chemical composition of uropygial gland waxes in domestic ducks. Biochem. Syst. Ecol. 7:149–153.
Ma, W. D., Wiesler, D., and Novotny, M. V. 1999. Urinary volatile profiles of the deermouse (Peromyscus maniculatus) pertaining to gender and age. J. Chem. Ecol. 25:417–431.
Manning, C. J., Wakeland, E. K., and Potts, W. K. 1992. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature 360:581–583.
Maynard Smith, J., and Harper, D. 2003. Animal Signals. Oxford University Press, Oxford.
Mclafferty, F. W. 1998. Wiley/NBS Registry of Mass Spectral Data, 4th ed. Wiley, New York, USA.
Mougeot, F., and Bretagnolle, V. 2000. Predation as a cost of sexual communication in nocturnal seabirds: an experimental approach using acoustic signals. Anim. Behav. 60:647–656.
Nevitt, G. A. and Bonadonna, F. 2005a. Seeing the world trough the nose of a bird: New developments in the sensory ecology of procellariiform seabirds. Marine Ecol. Prog. Ser. 287:292–295.
Nevitt, G. A. and Bonadonna, F. 2005b. Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biol. Lett. 1:303–305.
Roper, T. J. 1999. Olfaction in birds. Adv. Study Behav. 28:247–332.
Soini, H. A., Schrock, S. E., Bruce, K. E., Wiesler, D., Ketterson, E. D., and Novotny, M. V. 2007. Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J. Chem. Ecol. 33:183–198.
Stephen, S. 1998. NIST98 Mass Spectral Library. The NIST Mass Spectroscopy Data Center Gaithersburg, MD.
Stettenheim, P. 1972. The integument of birds, pp.1–63, in D. S. Farner, J. R. King (eds). Avian Biology. Academic, New York.
Warham, J. 1996. The Behaviour, Population Biology and Physiology of the Petrels, p. 613. Academic, London.
Wyatt, T. D. 2003. Pheromones and Animal Behaviour. Cambridge University Press, Cambridge.
Zelano, B., and Edwards, S. E. 2002. An Mhc component to kin recognition and mate choice in birds: predictions, progress, and prospects. Am. Nat. 160:225–238.
Acknowledgments
This study was supported by the Institut Polaire Français Paul Emile Victor (IPEV, Program no. 354) and performed in adherence to the IPEV/CNRS ethical guidelines. We thank B Buatois for the help with the gas chromatography; Dr. G Nevitt, C Bajzak, and Dr. S Caro for the help in the field; Prof. F S Dobson, Dr. P D’Ettorre, and Dr. M-C Anstett and two anonymous reviewers for the critical review of an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonadonna, F., Miguel, E., Grosbois, V. et al. Individual Odor Recognition in Birds: An Endogenous Olfactory Signature on Petrels’ Feathers?. J Chem Ecol 33, 1819–1829 (2007). https://doi.org/10.1007/s10886-007-9345-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9345-7