Abstract
This paper presents the synthesis of two cluster compounds {(NH4)2[Co(H2O)6]2V10O28·4H2O (C1) and (NH4)2[Ni(H2O)6]2V10O28·4H2O (C2)} which were obtained as single crystals suitable for XRD analysis that revealed their crystallization in the monoclinic (C2/c) and triclinic (P-1) space groups, respectively. Additionally, C1 and C2 were characterized using CHN analysis and FT-IR spectroscopy and their thermal decomposition mechanisms were investigated. The antibacterial activities of both compounds were determined against three human pathogenic bacterial strains {Bacillus cereus ATCC 33,018, Escherichia coli O157:H7 and Pseudomonas aeruginosa ATCC 9027} and one phytopathogenic bacterial strain {Ralstonia solanacearum}, while drug standards {chloramphenicol and streptomycin} were used as control. The inhibitory activity and the minimum inhibitory concentration (MIC) values of the tested compounds clearly indicated higher antibacterial activities of the nickel compound against B. cereus ATCC 33,018, E. coli O157 and R. solanacearum with MIC values of 3.150, 3.150 and 6.300 mg/ml, respectively. On the other hand, (NH4)2[Co(H2O)6]2V10O28·4H2O exhibited higher antibacterial activity against P. aeruginosa ATCC 9027 (MIC value of 6.300 mg/ml) in comparison to the nickel analog. In general, the measured activities are lower than that obtained for the standards except for the higher activity given by C2 in comparison to streptomycin against the R. solanacearum strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyoxometalates (POMs) are 3D negatively charged species of an accumulation of oxide clusters with a variety of transition metals, e.g. vanadium, molybdenum and tungsten [1]. These compounds are diversely applicable in electrochemistry [2], catalysis [3, 4], magnetochemistry [5], photochemistry [6] and medicine [7]. Pure inorganic POMs can show synergistic or direct antibacterial activity: Generally most of the inorganic POMs exhibit insignificant antibacterial activity at pharmacologically acceptable concentrations and the activities are enhanced in synergy with conventional antibiotics [8]. Nevertheless, twenty pure inorganic POMs were tested as direct antibacterial agents against six strains of Streptococcus pneumoniae and the polyoxovanadates (POVs) displayed high antibacterial activities whereas the other POMs that contained molybdenum and tungsten gave insignificant activities [9].
The polyoxovanadates (POVs), as a subclass of POMs, are divided into families of V(III), V(IV), V(V), V(IV)/V(III) and V(V)/V(IV) species and the family of V(V) clusters includes [V2O7]−4 [10], [V3O9]−3 [11], [V4O12]−4 [12], [V5O14]−3 [13], [V10O28]−6 [14], [V12O32]−4 [15], [V13O34]−3 [16], [V15O42]−9 [17] and [V16O42]−4 [18] species. The superb behavior of POVs is attributed to their structural diversity that permits numerous utilizations in medicine, biological processes, topology, geochemistry and energy-related applications [19,20,21].
The roles of vanadium-based compounds in biology are varied spanning from amending the activity of phosphatases [22], ATPase [23] and myosin [24] to inhibiting phosphofructokinase, hexokinase, adenylate kinase and tyrosine kinase [25,26,27]. This is in addition to the involvement of vanadium compounds as inorganic cofactors in alternative nitrogenases and haloperoxidases, and their relevance in the development of anticancer agents and insulin-mimetic agents [28,29,30,31]. The antibacterial activities of decavanadates, condensed {VO6} octahedra sharing vertices and edges [14], were reported against e.g. E. coli, K. pneumonia, S. marcescens, S. plymuthica and P. aeruginosa [32], and their activities were mostly attributed to the production of high amounts of reactive oxygen species [33].
On the other hand, cobalt and nickel are elements of superlative significance involved in biology. Cobalt is regarded as an essential element [34] and the applicability of several cobalt complexes is presumably due to their aqueous stability, availability and ease of synthesis [35] in addition to their in vivo insulin-like [36], anti-fungal and anti-oxidant [37] properties. Nickel is biologically involved in several enzymes, e.g. Ni–Fe hydrogenase, urease, acetyl-CoA decarbonylase/synthase, CO dehydrogenase, various superoxide dismutases, methyl coenzyme M reductase, methylenediurease, some glyoxylases and aci-reductonedioxygenase [38,39,40]. Various cobalt and nickel complexes have displayed activities against Gram-positive and Gram-negative bacteria, e.g. a cobalt complex of the anti-ulcer drug "famotidine" possessed antibacterial activities against M. lysodeikticus and E. coli higher than that of the free drug [41,42,43,44,45,46] and two complexes of nickel with amidodithiophosphonates showed good anti-proliferative activities against S. aureus and S. haemolyticus strains [47]. The combination of Co(II) or Ni(II) with V(V) systems is quite rare in biology and biomedical applications. Nevertheless, a recent study reported enhanced anti-bacterial activities for cobalt-vanadium oxides {Co3(VO4)2 and CoV2O6} in comparison to V2O5 [48] and another study confirmed that a synergistic reaction between Ni(II) and V(V) system resulted in impairing the microbial growth and survival [49].
This paper presents the synthesis and single-crystal structures of two cluster compounds, (NH4)2[Co(H2O)6]2V10O28·4H2O (C1) and (NH4)2[Ni(H2O)6]2V10O28·4H2O (C2), which in addition were studied by FT-IR spectroscopy and by thermal analysis (a group of techniques in which a property of the sample is monitored against time or temperature, and the temperature of the sample is programmed in a specific atmosphere). Taking into consideration the importance of introducing inorganic antibacterial agents in alternative to organic bactericides that usually suffer from a short life span and cause environmental pollution, we here assessed the antibacterial activities of C1 and C2 against three human pathogenic bacterial strains (B. cereus ATCC 33,018, E. coli O157:H7 and P. aeruginosa ATCC 9027) and one phytopathogenic bacterial strain (R. solanacearum), in comparison to reference drugs.
Materials and Methods
Physical Measurements
The starting chemicals, ammonium metavanadate, ammonium tartarate, cobalt chloride hexahydrate and nickel chloride hexahydrate, are analytical grade Alfa Aesar and Merck products and were used as received. For the synthesized compounds, the hydrogen and nitrogen contents were measured on Elementar Analysensysteme GmbH—vario EL III Element Analyzer and the infrared spectral data as KBr pellets were determined on a Nicolet iS10 spectrophotometer. The cluster compounds were studied by thermogravimetric (TG) analyses up to 500 °C in the air under the heating rate of 10 degrees/min on a Shimadzu DTG 60-H thermal analyzer. Selected crystals of compounds C1 and C2 were mounted on loops with protective oil and the X-ray data were collected on a Bruker APEX II diffractometer using graphite monochromated MoKα radiation (λ = 0.71073 Å) at 150(2) K operating in a φ and ω scans mode. The X-ray generator was operated at 50 kV and 30 mA and the data were monitored by the APEX [50] program. All data were corrected for Lorentzian, polarization and absorption effects using SAINT and SADABS [51] programs. SHELXT 2014 [52] and SHELXL 2014 [52] were respectively used for structure solution and for full matrix least-squares refinement on F2. These two programs are included in the WINGX-v2014.1 [53] program package. Non-hydrogen atoms were refined with anisotropic thermal parameters. The H-atoms of the water molecules and NH4 ions were located from the electron density map and allowed to refine freely. Molecular graphics, polyhedral representation of anion and packing diagrams, were drawn using Mercury 2020 [54]. Symmetry transformations {(-x + 1/2,-y + 3/2,-z + 1) and (-x + 1,-y,-z + 2)} were used to generate the equivalent atoms of the (V10O28)6− anion in C1 and C2, respectively. In (C1), a water molecule (O1S) was disordered over two positions with 0.5 occupancy factor.
Synthesis of the Clusters
For the synthesis of (NH4)2[Co(H2O)6]2V10O28·4H2O (C1) and (NH4)2[Ni(H2O)6]2V10O28·4H2O (C2), a mixture of ammonium metavanadate NH4VO3 (5.85 g, 50 mmol), ammonium tartrate (4.60 g, 25 mmol) and water (200 ml) was boiled under vigorous stirring until complete dissolution of the reagents. To this solution, the appropriate hexahydrated metal chloride (25 mmol; 5.94–5.95 g) was added with stirring. After stirring for 1 h at room temperature, any solids were removed by filtration. The products were recovered as single crystals by filtration from the reaction solutions after keeping them undisturbed at room temperature for a week. The crystals were washed with water and ethanol and dried in the air.
C1: Yield (478 mg, 6.8% based on vanadium). Anal. Calcd. (Found) % for Co2V10H40N2O44 (MW = 1399.58 g/mol) = H, 2.88 (3.01)% and N, 2.00 (2.13)%. FT-IR (KBr disk, cm−1): 3373 (br), 3191 (s), 1619 (s), 1420 (s), 961 (s), 822 (m), 741(m), 579 and 519 (m).
C2: Yield (1.43 g, 20.4% based on vanadium). Anal. Calcd. (Found) % for Ni2V10H40N2O44 (MW = 1399.10 g/mol) = H, 2.88 (3.05)% and N, 2.00 (2.14)%. FT-IR (KBr disk, cm−1): 3396 (br), 3171 (s), 1623 (s), 1418 (s), 947 (s), 823 (m), 740 (m), 579 (m) and 519 (m).
Antibacterial Activity
The antibacterial activity of the two cluster compounds was determined against three human pathogenic bacterial strains (B. cereus ATCC 33,018, E. coli O157:H7 and P. aeruginosa ATCC 9027) obtained from the American type culture collection and one locally isolated phytopathogenic bacterial strain (R. solanacearum). The inhibitory activity and the antibacterial agent lowest concentration that allows visible growth inhibition of the bacterial strains (minimum inhibitory concentrations, MIC) were determined by applying the broth dilution method [55]. In this method, bacterial strains are tested for their ability to generate visible growth in broth containing dilutions of the antimicrobial agent as follows: Twenty-four hours broth culture of each bacterial pathogen was performed by inoculation of a single colony into an Erlenmeyer flask containing 100 ml of trypticase soy broth and by incubation at 37 °C for B. cereus, E. coli and P. aeruginosa and at 30 °C for R. solanacearum. In the solidified Mueller–Hinton agar medium (Beef infusion: 300.0 g, Casein acid hydrolysate: 17.5 g, Starch: 1.5 g, Agar: 17.0 g, and 1000 ml of water) that permits good growth of most nonfastidious pathogens and is also low in antagonists, four wells of 8 mm diameter each were made using a sterilized cork borer and one hundred microliters from each culture were spread on the surface of the solidified agar medium. Five concentrations of each antibacterial agent were prepared in water and fifty microliters of each solution were added to each well, while chloramphenicol (for B. cereus, E. coli and P. aeruginosa) and streptomycin (for R. solanacearum) were added to the control plates. The antibacterial agents diffused into the agar medium and allowed for bacterial inhibition. The tests were performed in triplicate and the inhibition zone diameters (expressed in mm) were assessed after 48 h.
Results and Discussion
Chemistry
The polyoxovanadate based compounds, (NH4)2[Co(H2O)6]2V10O28∙4H2O (C1) and (NH4)2[Ni(H2O)6]2V10O28∙4H2O (C2), were isolated from reaction mixtures containing the desired hydrated metal chloride {CoCl2.6H2O/NiCl2.6H2O}, ammonium metavanadate and ammonium tartrate. All reagents were mixed in water at boiling temperature, before cooling the solutions to room temperature and allowing them to stand undisturbed for seven days. After this time, the clusters C1 and C2 were isolated as single crystals which exhibited air and light stability for months. In the reactions, every ten ions of \({\mathrm{VO}}_{3}^{-}\) polymerized to produce the cluster anion \({\mathrm{V}}_{10}{\mathrm{O}}_{28}^{6-}\) that attracted ammonium counter ions and hydrated cobalt and nickel ions for the assembly of the products. Indeed, the ammonium tartrate was added seeking the isolation of inorganic–organic clusters similar to others described in the literature [56]. However, the CHN analyses of the compounds indicated the absence of carbon revealing the absence of the tartrate anion in the products.
For the determination of the crystal structure of the products, single crystals of C1 (0.140 × 0.100 × 0.040 mm) and C2 (0.300 × 0.250 × 0.150 mm) were analyzed by XRD diffraction studies (Table 1). The compound C1 crystallized in monoclinic C2/c space group and the compound C2 crystallized in triclinic P-1 space group.
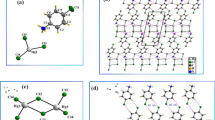
The asymmetric units contain one (NH4), two H2O, half (V10O28) and one [Co(H2O)6] for C1 or one [Ni(H2O)6] for C2. Figure 1 shows the asymmetric units of the solved structures extended by inversion centre {(C1)(-x + 1/2,-y + 3/2,-z + 1) and (C2)(-x + 1,-y,-z + 2)} to obtain the cluster anion \({\mathrm{V}}_{10}{\mathrm{O}}_{28}^{6-}\). Figure 2 displays the projection of the crystal packing, viewed along the [001] direction for compounds C1 and C2, showing the arrangement of \({\mathrm{V}}_{10}{\mathrm{O}}_{28}^{6-}\) anionic units isolated from each other by the cations {NH4 and Co(H2O)6 or Ni(H2O)6} and H2O molecules.
a Molecular structures of C1 (top) and C2 (bottom) with atomic numbering of the asymmetric units and thermal ellipsoids drawn at 40%, and b polyhedral model of decavanadate anions (V10O86−) showing the central plane formed by six vanadium atoms and the other four positioned two above and two below this plane
Selected bond distances for the structures of compounds C1 and C2 are listed in Table 2, while Table 3 includes selected angles for each structure. In compounds C1 and C2, the Co(II) and Ni(II) cations are six-coordinate with water oxygen atoms (O15-O20 in compound C1 and O16-O21 in compound C2). The Co(II) ion in C1 lies in the center of a slightly distorted octahedron with trans O—Co—O angles in the range 174.59(8)-176.59(8)° and Co—O distances in the range 2.077(2) Å—2.103(2) Å (average = 2.0875 Å). The corresponding angles and bond distances in compound C2 are in the ranges 172.84(9)-176.55(9)° and 2.040(3) Å—2.074(2) Å (average = 2.052 Å). In the decavanadate anions, the shortest V—V contacts are 3.04 and 3.052 Å in compounds C1 and C2, respectively. All V atoms are six-coordinate with the V—O bond distances varying according to the respective bond order. In the cluster anion \(\{{\mathrm{V}}_{10}{O}_{28}^{6-}\}\), four of the oxygen atoms are terminal (denoted as O1, O6, O9 and O11 in compound C1 and as O5, O7, O11 and O14 in compound C2), seven are μ2-bridged (denoted as O2, O3, O4, O5, O8, O10 and O14 in compound C1 and as O1, O2, O3, O4, O8, O12 and O15 in compound C2), two are μ3-bridged (denoted as O7 and O12 in compound C1 and as O9 and O10 in compound C2) and one is μ6-bridged (denoted as O13 in compound C1 and as O6 in compound C2). In compound C1, the terminal V = O bond distances are in the range 1.604(2) Å (V3—O9) to 1.628(2) Å (V2—O6)Å, the μ2(O)—V distances in the range 1.673(2) Å (O14—V5) to 2.097(1) Å (O14i—V3), the μ3(O)—V distances in the range 1.931(2) Å (O7—V5) to 2.029(1) Å (O12i —V2) and the μ6(O)—V distances in the range 2.116(1) Å (O13—V5) to 2.308(1) Å (O13i—V1). In its turn, the nickel compound C2 exhibits the following ranges of distances for the different types of V—O bonds: terminal V = O, 1.599(2) Å (V1—O5) to 1.610(2) Å (V2—O7); μ2(O)—V, 1.683(2) Å (O15i—V4) to 2.065(2) Å (O15—V5); μ3(O)—V, 1.904(2) Å (O10i—V4) to 2.003(2) Å (O10—V2); μ6(O)—V, 2.109(2) Å (O6i—V4) to 2.347(2) Å (O6—V1).
In both compounds, the oxygen atoms from the decavanadate anion act as acceptors in three-dimensional hydrogen bond networks with ammonium ions and H2O molecules as the donors (Table 4).
The FT-IR spectra of compound C1 and compound C2 are presented in Fig. 3. The sharp bands appearing in the spectra of the Co(II) and Ni(II) cluster compounds at 961 and 947 cm−1, respectively, are due to stretching vibrations υ(V = Ot) of the terminal oxygen-vanadium bonds (Ot = terminal oxygen) [57]. The asymmetric stretching vibrations of the bridging oxygen Ob – vanadium bonds υas(V—Ob) display frequencies of 822 and 741 cm−1 for compound C1 and 823 and 740 cm−1 for compound C2 [58,59,60]. The symmetric stretching vibrations of the same bonds υs(V—Ob) originate medium intensity bands at lower wavenumbers of 519 and 579 cm−1 for C1 and C2 [61]. The broad bands in the spectra of compound C1 and compound C2 appearing at frequencies around 3373 and 3396 cm−1 can be assigned to asymmetric and symmetric vibrations υ(O—H) of lattice water molecules [62, 63]. The sharp bands located at around 1619 and 1623 cm−1 in the spectra of compound C1 and compound C2, respectively, are due to the δ(H—O—H) bending vibrational modes of these water molecules [64]. The peaks at 3191 and 1420 cm−1 in the spectrum of C1 and at 3171 and 1418 cm−1 in the spectrum of C2 correspond, respectively, to symmetric and asymmetric stretching vibrations of the N—H bonds from the tetrahedral ammonium cation [60, 64].
Thermogravimetric analysis (thermal gravimetric analysis or TGA) is a technique in thermal analysis in which a change in the mass of a material is observed due to increase in temperature. The TGA curves of compounds C1 and C2 are presented in Fig. 4. Both compounds, when heated up to 500 °C, decomposed to stable residual products corresponding to 75.495 and 75.407% of the original mass, respectively. In detail, compound C1 decomposed in overlapped steps up to 264 °C (mass loss = 20.621%) in addition to a clear decomposition step up to the temperature of 373 °C (mass loss = 3.884%). Indeed, these weight losses are corresponding to the removal of sixteen solvated water molecules (Calcd. = 20.595%) and two ammonia (from the decomposition of the ammonium ions) and one extra water molecule (two protons from the ammonium ions and one oxygen atom from the decomposition of the decavanadate anion) (Calcd. = 3.721%). Therefore, the resulting residual product could be in the form of 2 Co(VO3)2 + 3 V2O5 (Calcd. = 75.684%). On the other hand, compound C2 started decomposing at about 110 °C, which is a relatively higher temperature than the decomposition temperature observed for compound C1. However, it also decomposed in an overlapped decomposition step up to the temperature of 275 °C and a clear decomposition step in the range of 275–391 °C. The weight losses found for C2 correspond to the removal of similar moieties as observed for the decomposition of compound C1 {in fact, the observed mass loss of 20.966% (Calcd. = 20.601%) corresponds also to the removal of sixteen water molecules and that of 3.627% (Calcd. = 3.723%) corresponds to the removal of one water and two ammonia molecules}. Therefore, the residual product is proposed to be a composite mixture of Ni(VO3)2 and V2O5 (2:3) (Calcd. = 75.676%).
Antibacterial Activity
The two cluster compounds, (NH4)2[Co(H2O)6]2V10O28·4H2O (C1) and (NH4)2[Ni(H2O)6]2V10O28·4H2O (C2), were tested for their antibacterial activities against four different human pathogens and phytopathogens representing Gram-positive and negative bacteria using the agar well diffusion assay method [55], with determination of inhibition zone diameters (Fig. 5) and minimum inhibitory concentrations (Table 5). Both compounds gave the same MIC value against P. aeruginosa ATCC 9027 and R. solanacearum, but C2 displayed lower MIC values against B. cereus ATCC 33,018 and E. coli O157. Generally, the results revealed antibacterial activity of the two compounds against the four tested bacterial pathogens. However, the nickel compound (C2) displayed higher activities than the cobalt counterpart (C1) against B. cereus ATCC 33,018, E. coli O157 and R. solanacearum with MIC values of 3.150, 3.150 and 6.300 mg/ml, respectively. On the other hand, the cobalt compound displayed higher activity against P. aeruginosa ATCC 9027 even if both compounds showed the same MIC concentration of 6.300 mg/ml. In numbers, C1 gave inhibition of 6 mm in the B. cereus culture only at a concentration of 12.60 mg/ml, while C2 at concentrations of 3.150, 4.201, 6.300 and 12.60 mg/ml afforded inhibitions respectively of 3, 5, 6 and 9 mm. As a control, chloramphenicol (10 mg/ml) displayed an inhibitory activity of 10 mm. On the other hand, compounds C1 and C2 displayed antibacterial activities against P. aeruginosa ATCC 9027 and R. solanacearum only at concentrations equal to or higher than 6.300 mg/ml. Compound C1 inhibited P. aeruginosa by 6 and 11 mm and C2 by 4 and 8 mm at concentrations of 6.300 and 12.60 mg/ml, respectively. In this strain, chloramphenicol (10 mg/ml) displayed an inhibitory activity of 12 mm. For the same concentrations (6.300 and 12.60 mg/ml) and against the R. solanacearum strain, C1 displayed inhibitory activities of 3 and 6 mm and C2 afforded inhibitions of 8 and 12 mm, while the positive control (streptomycin (10 mg/ml)) showed an inhibitory activity of 8 mm that is given by lower concentration of 6.300 mg/ml of C2 against R. solanacearum. Indeed, the least pronounced results were obtained against E. coli O157 for both compounds, as C1 and C2 showed activities not exceeding 1 mm for concentrations up to 12.60 mg/ml. In the same strain, chloramphenicol (10 mg/ml) displayed an inhibitory activity of 14 mm.
In fact, the activity difference between C1 and C2 is attributed to different activities of [Co(H2O)6]2+ and [Ni(H2O)6]2+ as these species interact differently with the active centers of the bacterium cell membrane. Additionally, this interaction could also be resulted from hydrogen bonding with the compounds due to the availability of donor and acceptor centers on the cluster compounds [65].
Conclusions
Two decavanadate based cluster compounds, (NH4)2[Co(H2O)6]2V10O28·4H2O (C1) and (NH4)2[Ni(H2O)6]2V10O28·4H2O (C2), were synthesized and their structures were determined by X-ray crystallography that revealed their stabilization through the formation of 3D networks based on extensive H-bonds. The compounds were further analyzed by FT-IR spectroscopy and thermal analysis that indicated overlapped steps of decomposition and decomposition to mixtures of vanadium (V) oxide and metal (II) vanadate M(VO3)2 (M = Co(II) or Ni(II)) at 500 °C. Both cluster compounds were tested as antibacterial agents against strains of different bacterial pathogens. The Ni cluster (C2) was more active against B. cereus ATCC 33,018, E. coli O157:H7 and R. solanacearum, while the P. aeruginosa ATCC 9027 strain was inhibited more intensively by the cobalt congener (C1). The antibacterial activities are lower than that of the standards, but C2 (6.300 mg/ml) and streptomycin (10 mg/ml) against R. solanacearum strains afforded the same inhibition of 8 mm.
References
D.-L. Long, R. Tsunashima, and L. Cronin (2010). Angew. Chem. Int. Ed. 49, 1736.
M. Sadakane and E. Steckhan (1998). Chem. Rev. 98, 219.
S.-S. Wang and G.-Y. Yang (2015). Chem. Rev. 115, 4893.
H. Lv, Y. V. Geletii, C. Zhao, J. W. Vickers, G. Zhu, Z. Luo, J. Song, T. Lian, D. G. Musaev, and C. L. Hill (2012). Chem. Soc. Rev. 41, 7572.
J. M. Clemente-Juan, E. Coronado, and A. Gaita-Ariño (2012). Chem. Soc. Rev. 41, 7464.
T. Ruther, V. M. Hultgren, B. P. Timko, A. M. Bond, W. R. Jackson, and A. G. Wedd (2003). J. Am. Chem. Soc. 125, 10133.
J. T. Rhule, C. L. Hill, D. A. Judd, and R. F. Schinazi (1998). Chem. Rev. 98, 327.
A. Bijelic, M. Aureliano, and A. Rompel (2018). Chem. Commun. 54 (10), 1153.
N. Fukuda and T. Yamase (1997). Biol. Pharm. Bull. 20, 927.
S. Aschwanden, H. W. Schmalle, A. Reller, and H. R. Oswald (1993). Mater. Res. Bull. 28, 575.
E. E. Hamilton, P. E. Fanwick, and J. J. Wilker (2002). J. Am. Chem. Soc. 124, 78.
J. Li, C. Wei, D. GuO, C. Wang, Y. Han, G. He, J. Zhang, X. Huang, and C. Hu (2020). Dalton Trans. 49, 14148.
V. W. Day, W. G. Klemperer, and O. M. Yaghi (1989). J. Am. Chem. Soc. 111, 4518.
E. Sánchez-Lara, A. Pérez-Benítez, S. Treviño, A. Mendoza, F. Meléndez, E. Sánchez-Mora, S. Bernès, and E. González-Vergara (2016). Crystals 6, 65.
T. Kurata, Y. Hayashi, and K. Isobe (2010). Chem. Lett. 39, 708.
D. Hou, K. D. Hagen, and C. L. Hill (1992). J. Am. Chem. Soc. 114, 5864.
D. Hou, K. S. Hagen, and C. L. Hill (1993). J. Chem. Soc. Chem. Commun. 4, 426.
J. Marrot, K. Barthelet, C. Simonnet, and D. Riou (2005). C.R. Chim. 8, 971.
M. Aureliano (2009). Dalton Trans. 42, 9093.
A. Gorzsás, I. Andersson, and L. Pettersson (2006). Eur. J. Inorg. Chem. 18, 3559.
A. Xie, C.-A. Ma, L. Wang, and Y. Chu (2007). Electrochim. Acta 52, 2945.
T. L. Turner, V. H. Nguyen, C. C. McLauchlan, Z. Dymon, B. M. Dorsey, J. D. Hooker, and M. A. Jones (2012). J. Inorg. Biochem. 108, 96.
S. Hua, G. Inesi, and C. Toyoshima (2000). J. Biol. Chem. 275, 30546.
T. Tiago, M. Aureliano, and C. Gutierrez-Merino (2004). Biochemistry 43, 5551.
E. G. Demaster and R. A. Mitchell (1973). Biochemistry 12, 3616.
G. Soman, Y. C. Chang, and D. J. Graves (1983). Biochemistry 22, 4994.
M. Zhao, X. Chen, G. Chi, D. Shuai, L. Wang, B. Chen, and J. Li (2020). Inorg. Chem. Front. 7, 4320.
B. Mukherjee, B. Patra, S. Mahapatra, P. Banerjee, A. Tewari, and M. Chatterjee (2004). Toxicol. Lett. 150, 135.
D. G. Barceloux (1999). J. Toxicol. Clin. Toxicol. 37, 265.
F. Zhai, X. Wang, D. Li, H. Zhang, R. Li, and L. Song (2009). Biomed. Pharmacother. 63, 51.
V. Dimitrova, K. Zhetcheva, and L. P. Pavlova (2011). J. Chem. 35, 215.
G.A.-E. Mahmoud, A. B. M. Ibrahim, and P. Mayer (2021). ChemistrySelect 6 (15), 3782.
D. Rehder (2013). Dalton Trans. 42, 11749.
S. Hatamie, M. Nouri, S. K. Karandikar, A. Kulkarni, S. D. Dhole, and D. M. Phase (2012). Mater Sci. Eng. C 32, 92.
E. L. Chang, C. Simmers, and D. A. Knight (2010). Pharmaceuticals 3, 1711.
L. Yang, D. C. Crans, S. M. Miller, A. la Cour, O. P. Anderson, P. M. Kaszynski, M. E. Godzala, L. D. Austin, and G. R. Willsky (2002). Inorg. Chem. 41, 4859.
F. Dimiza, A. N. Papadopoulos, V. Tangoulis, V. Psycharis, C. P. Raptopoulou, D. P. Kessissoglou, and G. Psomas (2010). Dalton Trans. 39, 4517.
R. Bartha and E. J. Ordal (1965). J. Bacteriol. 89, 1015.
R. P. Hausinger (1987). Microbiol. Rev. 51, 22.
G. Fierros-Romero, J. A. Wrosek-Cabrera, M. Gomez-Ramırez, R. C. Pless, A. M. Rivas-Castillo, and N. G. Rojas-Avelizapa (2017). Curr. Microbiol. 74, 840.
D. U. Miodragovic, G. A. Bogdanovic, Z. M. Miodragovic, M. D. Radulovic, S. B. Novakovic, G. N. Kaluderovic, and H. Kozlowski (2006). J. Inorg. Biochem. 100, 1568.
K. Nomiya, A. Yoshizawa, K. Tsukagoshi, N. C. Kasuga, S. Hirakawa, and J. Watanabe (2004). J. Inorg. Biochem. 98, 46.
M. C. Rodriguez-Argüelles, S. Mosquera-Vazquez, J. Sanmartin-Matalobos, A. M. Garcia-Deibe, C. Pelizzi, and F. Zani (2010). Polyhedron 29, 864.
K. Matsumoto, S. Yamamoto, Y. Yoshikawa, M. Doe, Y. Kojima, H. Sakurai, H. Hashimoto, and M. N. Kajiwara (2005). Bull. Chem. Soc. Jpn. 78, 1077.
P. Dorkov, I. N. Pantcheva, W. S. Sheldrick, H. Mayer-Figge, R. Petrova, and M. Mitewa (2008). J. Inorg. Biochem. 102, 26.
J. Lv, T. Liu, S. Cai, X. Wang, L. Liu, and Y. Wang (2006). J. Inorg. Biochem. 100, 1888.
E. Podda, M. Arca, G. Atzeni, S. J. Coles, A. Ibba, F. Isaia, V. Lippolis, G. Orrù, J. B. Orton, A. Pintus, E. Tuveri, and M. C. Aragoni (2020). Molecules 25, 2052.
A. Simo, M. Drah, N. R. S. Sibuyi, M. Nkosi, M. Meyer, and M. Maaza (2018). Ceram. Int. 44 (7), 7716.
I. Kamika and M. N. B. Momba (2013). Desalination Water Treat. 51, 7431.
Bruker, SMART and SAINT (2008) Bruker AXS Inc., Madison, WI
G. M. Sheldrick, SADABS (Bruker AXS Inc., Madison, WI, 2004).
G. M. Sheldrick (2015). Acta Crystallogr. 71, 3.
L. J. Farrugia (1999). J. Appl. Crystallogr. 32, 837.
C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. J. Taylor, J. Van De Streek, and P. A. Wood (2008). J. Appl. Crystallogr. 41, 466.
I. Wiegand, K. Hilpert, and R. E. W. Hancock (2008). Nat. Protoc. 3 (2), 163.
L. Klistincova, E. Rakovsky, and P. Schwendt (2010). Transit. Met. Chem. 35, 229.
S. Du, N. Zho, and X. Wu (1994). Polyhedron 13, 301.
C. Li, D. Zhong, X. Huang, G. Shen, Q. Li, J. Du, Q. Li, S. Wang, J. Li, and J. Dou (2019). New J. Chem. 43, 5813.
S. Li, Z. Li, J. Zhang, Z. Su, S. Qi, S. Guo, and X. Tan (2017). J. Chem. Sci. 129 (5), 573.
L. Krivosudsky, A. Roller, and A. Rompel (2019). New J. Chem. 43, 17863.
E. Sánchez-Lara, I. Sánchez-Lombardo, A. Pérez-Benítez, Á. Mendoza, M. Flores-Álamo, and E. G. Vergara (2015). J. Clust. Sci. 26, 901.
Z. J. Zhong, X. Z. You, and Q. C. Yang (1994). Polyhedron 13, 1951.
X. Li, L. Yan, H. Wanyan, and R. Yang (1993). Polyhedron 13, 2021.
I. Mestiri, B. Ayed, and A. Haddad (2013). J. Clust. Sci. 24, 85.
E. Feacium, S. Toumi, N. Ratel-Ramond, and S. Akriche (2015). J. Clust. Sci. 26, 1821.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mamdouh, AA., Ibrahim, A.B.M., Reyad, N.EH.A. et al. (NH4)2[Co(H2O)6]2V10O28·4H2O Vs. (NH4)2[Ni(H2O)6]2V10O28·4H2O: Structural, Spectral and Thermal Analyses and Evaluation of Their Antibacterial Activities. J Clust Sci 34, 1535–1546 (2023). https://doi.org/10.1007/s10876-022-02326-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02326-2