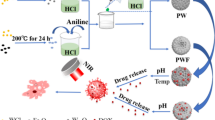
Abstract
Near infrared (NIR)-triggered photothermal therapy (PTT) usually requires hyperthermia for effective tumor therapy, leading to side effects such as overheating damage. It is critical and challenging for the successful clinical application of PTT through achieving effective tumor therapy under mild temperature. Herein, a joint strategy of autophagy inhibition and lysosomal escape based on PEG-PEI/CDs-E64d nanoagents was designed using PEG as a carrier to combine PEI with “proton sponge” effect and autophagy inhibitor of E64d, which improved the sensitivity of tumor cells to treatment and the utilization of photothermal agent (carbon quantum dots, CDs), and were characterized in detail by transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS), Zeta, Western blot, etc. The excellent photothermal effect, autophagy inhibition and lysosomal escape ability were proved in vitro experiments, and the results of in vivo experiment showed the effective and safe low-temperature PTT effect was achieved with tumor inhibition rate of 84.3% for 9 days of treatment at 42 °C. In general, the joint strategy we proposed provides a useful exploration for the clinical application of PTT.
Graphical Abstract










Similar content being viewed by others
References
J. Kim, Y. M. Lee, Y. Kang, and W. J. Kim (2014). Tumor-homing, size-tunable clustered nanoparticles for anticancer therapeutics. ACS Nano. 8 (9), 9358–9367. https://doi.org/10.1021/nn503349g.
B. P. Timko, T. Dvir, and D. S. Kohane (2010). Remotely triggerable drug delivery systems. Adv. Mater. 22 (44), 4925–4943. https://doi.org/10.1002/adma.201002072.
Q. Chen, C. Liang, X. Wang, J. He, Y. Li, and Z. Liu (2014). An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials. 35 (34), 9355–9362. https://doi.org/10.1016/j.biomaterials.2014.07.062.
R. Xiong, D. Hua, J. Van Hoeck, D. Berdecka, L. Leger, S. De Munter, J. C. Fraire, L. Raes, A. Harizaj, F. Sauvage, G. Goetgeluk, M. Pille, J. Aalders, J. Belza, T. Van Acker, E. Bolea-Fernandez, T. Si, F. Vanhaecke, W. H. De Vos, B. Vandekerckhove, J. van Hengel, K. Raemdonck, C. Huang, S. C. De Smedt, and K. Braeckmans (2021). Photothermal nanofibres enable safe engineering of therapeutic cells. Nat. Nanotechnol. 16 (11), 1281–1291. https://doi.org/10.1038/s41565-021-00976-3.
X. Y. Qu, Y. Hong, H. Cai, X. Sun, Q. Shen, D. L. Yang, X. C. Dong, A. H. Jiao, P. Chen, and J. J. Shao (2022). Promoted intramolecular photoinduced-electron transfer for multi-mode imaging-guided cancer photothermal therapy. Rare. Met. 41 (1), 56–66. https://doi.org/10.1007/s12598-021-01795-0.
Z. Zhang, J. Wang, and C. Chen (2013). Near-Infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv. Mater. 25 (28), 3869–3880. https://doi.org/10.1002/adma.201301890.
X. Wang, J. Zhang, Y. Wang, C. Wang, J. Xiao, Q. Zhang, and Y. Cheng (2016). Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterial. 81, 114–124. https://doi.org/10.1016/j.biomaterials.2015.11.037.
G. Liu, J. Zou, Q. Tang, X. Yang, Y. Zhang, Q. Zhang, W. Huang, P. Chen, J. Shao, and X. Dong (2017). Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS. Appl. Mater. Interfaces. 9 (46), 40077–40086. https://doi.org/10.1021/acsami.7b13421.
J. Gao, C. Wu, D. Deng, P. Wu, and C. Cai (2016). Direct synthesis of water-soluble aptamer-Ag2S quantum dots at ambient temperature for specific imaging and photothermal therapy of cancer. Adv. Healthc. Mater. 5 (18), 2437–2449. https://doi.org/10.1002/adhm.201600545.
D. Yoo, H. Jeong, S. H. Noh, J. H. Lee, and J. Cheon (2013). Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew. Chem. Int. Ed. 52 (49), 13047–13051. https://doi.org/10.1002/anie.201306557.
N. Mizushima, B. Levine, A. M. Cuervo, and D. J. Klionsky (2008). Autophagy fights disease through cellular self-digestion. Nature. 451 (7182), 1069–1075. https://doi.org/10.1038/nature06639.
G. Gao, X. Sun, X. Liu, Y. W. Jiang, R. Tang, Y. Guo, F. G. Wu, and G. Liang (2021). Intracellular nanoparticle formation and hydroxychloroquine release for autophagy-inhibited mild-temperature photothermal therapy for tumors. Adv. Funct. Mater. 31 (34), 2102832. https://doi.org/10.1002/adfm.202102832.
G. Gao, Y. W. Jiang, Y. Guo, H. R. Jia, X. Cheng, Y. Deng, X. W. Yu, Y. X. Zhu, H. Y. Guo, W. Sun, X. Liu, J. Zhao, S. Yang, Z. W. Yu, F. M. S. Raya, G. Liang, and F. G. Wu (2020). Enzyme-mediated tumor starvation and phototherapy enhance mild-temperature photothermal therapy. Adv. Funct. Mater. 30 (16), 1909391. https://doi.org/10.1002/adfm.201909391.
G. Gao, Y. W. Jiang, W. Sun, Y. Guo, H. R. Jia, X. W. Yu, G. Y. Pan, and F. G. Wu (2019). Molecular targeting-mediated mild-temperature photothermal therapy with a smart albumin-based nanodrug. Small. 15 (33), 1900501. https://doi.org/10.1002/smll.201900501.
X. Yi, Q. Y. Duan, and F. G. Wu (2021). Low-Temperature photothermal therapy: strategies and applications. Research. 2021, 9816594. https://doi.org/10.34133/2021/9816594.
H. Chaachouay, P. Ohneseit, M. Toulany, R. Kehlbach, G. Multhoff, and H. P. Rodemann (2011). Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother. Oncol. 99 (3), 287–292. https://doi.org/10.1016/j.radonc.2011.06.002.
M. F. Wei, M. W. Chen, K. C. Chen, P. J. Lou, S. Y. F. Lin, S. C. Hung, M. Hsiao, C. J. Yao, and M. J. Shieh (2014). Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 10 (7), 1179–1192. https://doi.org/10.4161/auto.28679.
J. Li, N. Hou, A. Faried, S. Tsutsumi, and H. Kuwano (2010). Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur. J. Cancer. 46 (10), 1900–1909. https://doi.org/10.1016/j.ejca.2010.02.021.
M. E. Davis, Z. Chen, and D. M. Shin (2008). Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 7 (9), 771–782. https://doi.org/10.1038/nrd2614.
G. Kroemer, G. Marino, and B. Levine (2010). Autophagy and the integrated stress response. Mol. Cell. 40 (2), 280–293. https://doi.org/10.1016/j.molcel.2010.09.023.
J. A. Rudnick, T. Monkkonen, F. A. Mar, J. M. Barnes, H. Starobinets, J. Goldsmith, S. Roy, S. B. Eguiguren, V. M. Weaver, and J. Debnath (2021). Autophagy in stromal fibroblasts promotes tumor desmoplasia and mammary. Gene. Dev. 35 (13–14), 963–975.
P. M. Yang, Y. L. Liu, Y. C. Lin, C. T. Shun, M. S. Wu, and C. C. Chen (2010). Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer. Res. 70 (19), 7699–7709. https://doi.org/10.1158/0008-5472.can-10-1626.
Z. G. Movahed, R. Yarani, P. Mohammadi, and K. Mansouri (2021). Sustained oxidative stress instigates differentiation of cancer stem cells into tumor endothelial cells: pentose phosphate pathway, reactive oxygen species and autophagy crosstalk. Biomed. Pharmacother. 139, 11643. https://doi.org/10.1016/j.biopha.2021.111643.
Z. Zhou, Y. Yan, K. Hu, Y. Zou, Y. Li, R. Ma, Q. Zhang, and Y. Cheng (2017). Autophagy inhibition enabled efficient photothermal therapy at a mild temperature. Biomaterials. 141, 116–124. https://doi.org/10.1016/j.biomaterials.2017.06.030.
X. Zhang, X. Zeng, X. Liang, Y. Yang, X. Li, H. Chen, L. Huang, L. Mei, and S. S. Feng (2014). The chemotherapeutic potential of PEG-b-PLGA copolymer micelles that combine chloroquine as autophagy inhibitor and docetaxel as an anti-cancer drug. Biomaterials. 35 (33), 9144–9154. https://doi.org/10.1016/j.biomaterials.2014.07.028.
Y. Xu, H. Yu, H. Qin, J. Kang, C. Yu, J. Zhong, J. Su, H. Li, and L. Sun (2012). Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett. 314 (2), 232–243. https://doi.org/10.1016/j.canlet.2011.09.034.
M. Tamai, K. Matsumoto, S. Omura, I. Koyama, Y. Ozawa, and K. Hanada (1986). In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J. Pharmacobio-Dyn. 9 (8), 672–677. https://doi.org/10.1248/bpb1978.9.672.
I. Tanida, N. Minematsu-Ikeguchi, T. Ueno, and E. Kominami (2005). Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 1 (2), 84–91. https://doi.org/10.4161/auto.1.2.1697.
M. Wan, H. Chen, Q. Wang, Q. Niu, P. Xu, Y. Yu, T. Zhu, C. Mao, and J. Shen (2019). Bio-inspired nitric-oxide-driven nanomotor. Nat. Commun. 10 (1), 1–11. https://doi.org/10.1038/s41467-019-08670-8.
L. Rao, L. L. Bu, B. Cai, J. H. Xu, A. Li, W. F. Zhang, Z. J. Sun, S. S. Guo, W. Liu, T. H. Wang, and X. Z. Zhao (2016). Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. 28 (18), 3460–3466. https://doi.org/10.1002/adma.201506086.
N. Bertrand, J. Wu, X. Xu, N. Kamaly, and O. C. Farokhzad (2014). Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug. Delivery. Rev. 66, 2–25. https://doi.org/10.1016/j.addr.2013.11.009.
G. Hartmann, R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg (2000). Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164 (3), 1617–1624. https://doi.org/10.4049/jimmunol.164.3.1617.
X. Sun, X. Yan, O. Jacobson, W. Sun, Z. Wang, X. Tong, Y. Xia, D. Ling, and X. Chen (2017). Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics. 7(2), 319–328. https://www.thno.org/v07p0319.htm.
M. R. Horsman and P. Vaupel (2016). Pathophysiological Basis for the Formation of the Tumor Microenvironment. Front. Oncol. 6, 66. https://doi.org/10.3389/fonc.2016.00066.
Z. Guo, M. Chen, C. Peng, S. Mo, C. Shi, G. Fu, X. Wen, R. Zhuang, X. Su, T. Liu, N. Zheng, and X. Zhang (2018). pH-sensitive radiolabeled and superfluorinated ultra-small palladium nanosheet as a high-performance multimodal platform for tumor theranostics. Biomaterials. 179, 134–143. https://doi.org/10.1016/j.biomaterials.2018.06.040.
M. M. Wan, T. T. Xu, B. Chi, M. Wang, Y. Huang, Q. Wang, T. Li, W. Q. Yan, H. Chen, P. Xu, C. Mao, B. Zhao, J. Shen, H. Xu, and D. Q. Shi (2019). A safe and efficient strategy for the rapid elimination of blood lead in vivo based on a capture-fix-separate mechanism. Angew. Chem. Int. Ed. 58 (31), 10582–10586. https://doi.org/10.1002/anie.201904044.
D. Kliosnky, et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 12, 1–222. https://doi.org/10.1080/15548627.2015.1100356.
Z. Wan, H. Mao, M. Guo, Y. Li, A. Zhu, H. Yang, H. He, J. Shen, L. Zhou, Z. Jiang, C. Ge, X. Chen, X. Yang, G. Liu, and H. Chen (2014). Highly efficient hierarchical micelles integrating photothermal therapy and singlet oxygen-synergized chemotherapy for cancer eradication. Theranostics. 4(4), 399–411. https://www.thno.org/v04p0399.htm.
S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer, and W. C. W. Chan (2009). Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9 (5), 1909–1915. https://doi.org/10.1021/nl900031y.
D. Jaque, L. Martinez Maestro, B. del Rosal, P. Haro Gonzalez, A. Benayas, J. L. Plaza, E. Martin Rodriguez, and J. Garcia Sole (2014). Nanoparticles for photothermal therapies. Nanoscale. 6 (16), 9494–9530. https://doi.org/10.1039/c4nr00708e.
L. C. Kennedy, L. R. Bickford, N. A. Lewinski, A. J. Coughlin, Y. Hu, E. S. Day, J. L. West, and R. A. Drezek (2011). A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 7 (2), 169–183. https://doi.org/10.1002/smll.201000134.
F. Janku, D. J. McConkey, D. S. Hong, and R. Kurzrock (2011). Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 8 (9), 528–539. https://doi.org/10.1038/nrclinonc.2011.71.
B. Wang, H. Song, X. Qu, J. Chang, B. Yang, and S. Lu (2021). Carbon dots as a new class of nanomedicines: Opportunities and challenges. Coord. Chem. Rev. 442, 214010. https://doi.org/10.1016/j.ccr.2021.214010.
H. Yu, X. Lv, L. Wu, B. Li, K. Huang, Y. Huang, Q. Zhang, C. Mei, X. Ren, R. Zhou, H. Luo, P. Pang, and H. Shan (2020). Doxorubicin-loaded fluorescent carbon dots with PEI passivation as a drug delivery system for cancer therapy. Nanoscale. 12 (33), 17222–17237. https://doi.org/10.1039/d0nr01236j.
D. Pei and M. Buyanova (2019). Overcoming endosomal entrapment in drug delivery. Bioconjugate Chem. 30 (2), 273–283. https://doi.org/10.1021/acs.bioconjchem.8b00778.
S. Marrache and S. Dhar (2012). Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. P. Natl. Acad. Sci. USA 109 (40), 16288–16293. https://doi.org/10.1073/pnas.1210096109.
C. Yang, W. Cheng, P. Y. Teo, A. C. Engler, D. J. Coady, J. L. Hedrick, and Y. Y. Yang (2013). Mitigated cytotoxicity and tremendously enhanced gene transfection efficiency of PEI through facile one-step carbamate modification. Adv. Healthcare. Mater. 2 (10), 1304–1308. https://doi.org/10.1002/adhm.201300046.
X. Tu, L. Wang, Y. Cao, Y. Ma, H. Shen, M. Zhang, and Z. Zhang (2016). Efficient cancer ablation by combined photothermal and enhanced chemo-therapy based on carbon nanoparticles/doxorubicin@SiO2 nanocomposites. Carbon. 97, 35–44. https://doi.org/10.1016/j.carbon.2015.05.043.
H. Wang, J. Di, Y. Sun, J. Fu, Z. Wei, H. Matsui, and A. d. C. Alonso, and S. Zhou, (2015). Biocompatible PEG-Chitosan@Carbon dots hybrid nanogels for two-photon fluorescence imaging, Near-Infrared light/pH dual-responsive drug carrier, and synergistic therapy. Adv. Funct. Mater. 25 (34), 5537–5547. https://doi.org/10.1002/adfm.201501524.
K. Han, W. Y. Zhang, J. Zhang, Q. Lei, S. B. Wang, J. W. Liu, X. Z. Zhang, and H. Y. Han (2016). Acidity-triggered tumor-targeted chimeric peptide for enhanced intra-nuclear photodynamic therapy. Adv. Funct. Mater. 26 (24), 4351–4361. https://doi.org/10.1002/adfm.201600170.
X. Li, R. Wu, H. Chen, T. Li, H. Jiang, X. Xu, X. Tang, M. Wan, C. Mao, and D. Shi (2021). Near-Infrared Light-Driven Multifunctional Tubular Micromotors for Treatment of Atherosclerosis. Acs Appl. Mater. Interfaces. 13 (26), 30930–30940. https://doi.org/10.1021/acsami.1c03600.
M. Wan, Q. Wang, R. Wang, R. Wu, T. Li, D. Fang, Y. Huang, Y. Yu, L. Fang, X. Wang, Y. Zhang, Z. Miao, B. Zhao, F. Wang, C. Mao, Q. Jiang, X. Xu, and D. Shi (2020). Platelet-derived porous nanomotor for thrombus therapy. Sci. Adv. 6(22), eaaz9014.
U. Prabhakar, H. Maeda, R. K. Jain, E. M. Sevick-Muraca, W. Zamboni, O. C. Farokhzad, S. T. Barry, A. Gabizon, P. Grodzinski, and D. C. Blakey (2013). Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 73 (8), 2412–2417. https://doi.org/10.1158/0008-5472.can-12-4561.
B. A. Chabner and T. G. Roberts (2005). Timeline chemotherapy and the war on cancer. Nat. Rev. Cancer. 5 (1), 65–72. https://doi.org/10.1038/nrc1529.
H. Maeda (2015). Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliver. Rev. 91, 3–6. https://doi.org/10.1016/j.addr.2015.01.002.
H. Wang, Z. Wei, H. Matsui, and S. Zhou (2014). Fe3O4/carbon quantum dots hybrid nanoflowers for highly active and recyclable visible-light driven photocatalyst. J. Mater. Chem. A. 2 (38), 15740–15745. https://doi.org/10.1039/c4ta03130j.
D. J. Stewart, R. Konnan, T. A. Grusenmeyer, J. M. Artz, S. L. Long, Z. Yu, T. M. Cooper, J. E. Haley, and L. S. Tan (2018). Effects of intramolecular hydrogen bonding and sterically forced non-coplanarity on organic donor/acceptor two-photon-absorbing molecules. Phys. Chem. Chem. Phys. 20 (29), 19398–19407. https://doi.org/10.1039/c8cp02647e.
R. P. Velasco, U. Chaikledkaew, C. Y. Myint, R. Khampang, S. Tantivess, and Y. Teerawattananon (2013). Advanced health biotechnologies in Thailand: redefining policy directions. J. Transl. Med. 11, 1. https://doi.org/10.1186/1479-5876-11-1.
D. M. Waag, M. J. McCluskie, N. L. Zhang, and A. M. Krieg (2006). A CpG oligonucleotide can protect mice from a low aerosol challenge dose of Burkholderia mallei. Infect. Immun. 74 (3), 1944–1948. https://doi.org/10.1128/iai.74.3.1944-1948.2006.
P. Wu, D. Deng, J. Gao, and C. Cai (2016). Tubelike gold sphere-attapulgite nanocomposites with a high photothermal conversion ability in the Near-Infrared region for enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces. 8 (16), 10243–10252. https://doi.org/10.1021/acsami.6b02270.
Y. Liu, K. Ai, J. Liu, M. Deng, Y. He, and L. Lu (2013). Dopamine-melanin colloidal nanospheres: an efficient Near-Infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 25 (9), 1353–1359. https://doi.org/10.1002/adma.201204683.
D. K. Roper, W. Ahn, and M. Hoepfner (2007). Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C. 111 (9), 3636–3641. https://doi.org/10.1021/jp064341w.
H. Duan and S. Nie (2007). Cell penetrating quantum dots based on multivalent and endosome disrupting surface coatings. J. Am. Chem. Soc. 129 (11), 3333–3338. https://doi.org/10.1021/ja068158s.
R. T. Lima, D. Sousa, A. M. Paiva, A. Palmeira, J. Barbosa, M. Pedro, M. M. Pinto, E. Sousa, and M. H. Vasconcelos (2016). Modulation of autophagy by a thioxanthone decreases the viability of melanoma cells. Molecules. 21 (10), 1343. https://doi.org/10.3390/molecules21101343.
Acknowledgements
The work was supported by National Natural Science Foundation of China (No.: 22175096), Social Development Project of Jiangsu Natural Science Foundation (No.: BE2019744), Collaborative Innovation Center of Biomedical Functional Materials, the Priority Academic Program Development of Jiangsu Higher Education Institution.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, J., Zhang, Y. et al. Joint Strategy of PEG-PEI/CDs-E64d Nanoagents for Effective Low-Temperature Photothermal Therapy. J Clust Sci 34, 865–880 (2023). https://doi.org/10.1007/s10876-022-02262-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02262-1